NMR Spectroscopy of the Main Protease of SARS-CoV-2 and Fragment-Based Screening Identify Three Protein Hotspots and an Antiviral Fragment.
Cantrelle, F.X., Boll, E., Brier, L., Moschidi, D., Belouzard, S., Landry, V., Leroux, F., Dewitte, F., Landrieu, I., Dubuisson, J., Deprez, B., Charton, J., Hanoulle, X.(2021) Angew Chem Int Ed Engl 60: 25428-25435
- PubMed: 34570415
- DOI: https://doi.org/10.1002/anie.202109965
- Primary Citation of Related Structures:
7NTS, 7P51 - PubMed Abstract:
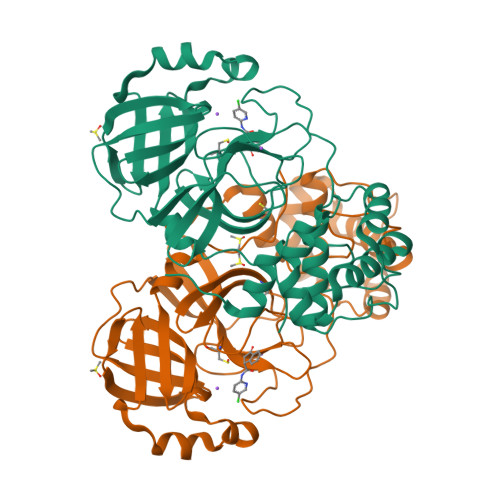
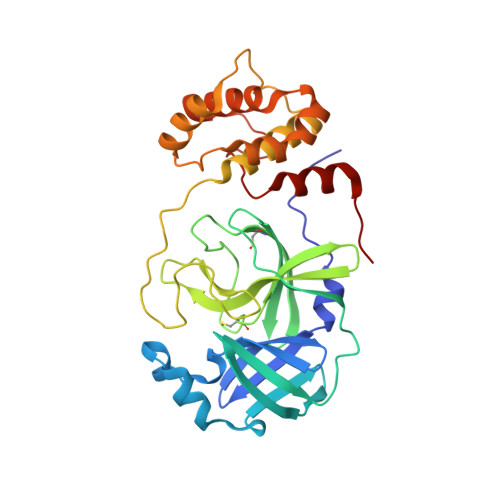
The main protease (3CLp) of the SARS-CoV-2, the causative agent for the COVID-19 pandemic, is one of the main targets for drug development. To be active, 3CLp relies on a complex interplay between dimerization, active site flexibility, and allosteric regulation. The deciphering of these mechanisms is a crucial step to enable the search for inhibitors. In this context, using NMR spectroscopy, we studied the conformation of dimeric 3CLp from the SARS-CoV-2 and monitored ligand binding, based on NMR signal assignments. We performed a fragment-based screening that led to the identification of 38 fragment hits. Their binding sites showed three hotspots on 3CLp, two in the substrate binding pocket and one at the dimer interface. F01 is a non-covalent inhibitor of the 3CLp and has antiviral activity in SARS-CoV-2 infected cells. This study sheds light on the complex structure-function relationships of 3CLp and constitutes a strong basis to assist in developing potent 3CLp inhibitors.
Organizational Affiliation:
CNRS ERL9002-BSI-Integrative Structural Biology, 50 avenue Halley, F-59658 Villeneuve d'Ascq, Lille, France.