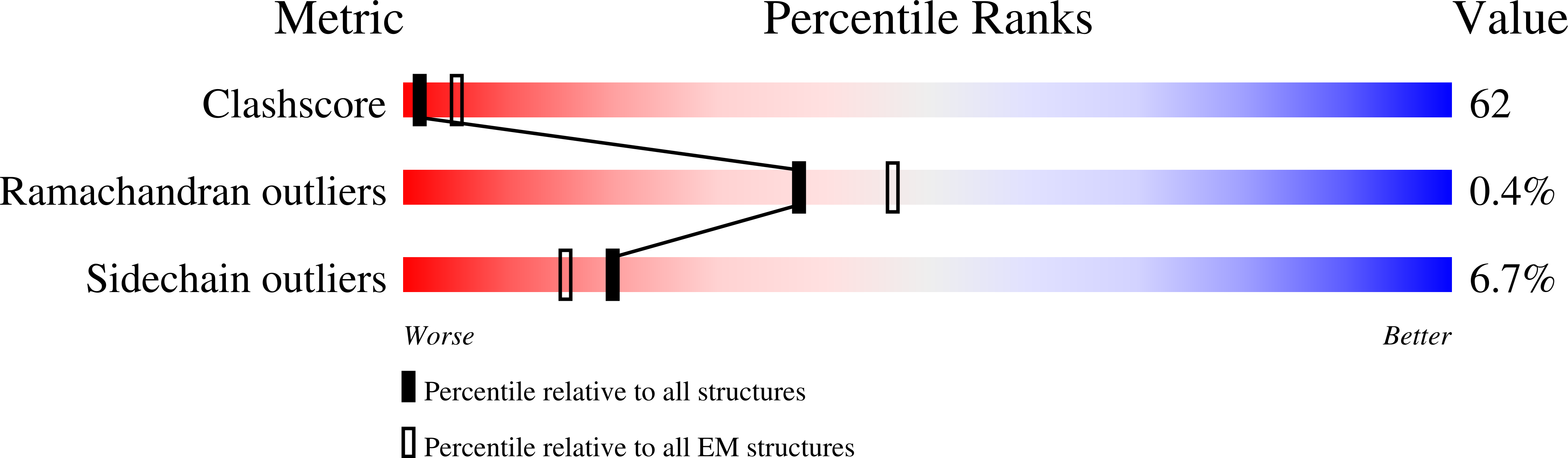The structural role of SARS-CoV-2 genetic background in the emergence and success of spike mutations: The case of the spike A222V mutation.
Ginex, T., Marco-Marin, C., Wieczor, M., Mata, C.P., Krieger, J., Ruiz-Rodriguez, P., Lopez-Redondo, M.L., Frances-Gomez, C., Melero, R., Sanchez-Sorzano, C.O., Martinez, M., Gougeard, N., Forcada-Nadal, A., Zamora-Caballero, S., Gozalbo-Rovira, R., Sanz-Frasquet, C., Arranz, R., Bravo, J., Rubio, V., Marina, A., Geller, R., Comas, I., Gil, C., Coscolla, M., Orozco, M., Llacer, J.L., Carazo, J.M.(2022) PLoS Pathog 18: e1010631-e1010631
- PubMed: 35816514
- DOI: https://doi.org/10.1371/journal.ppat.1010631
- Primary Citation of Related Structures:
7QDG, 7QDH - PubMed Abstract:
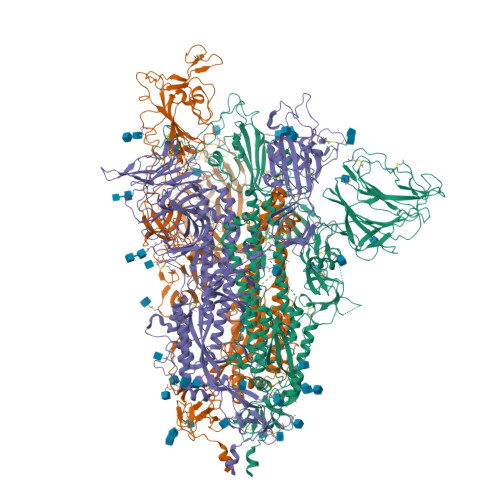
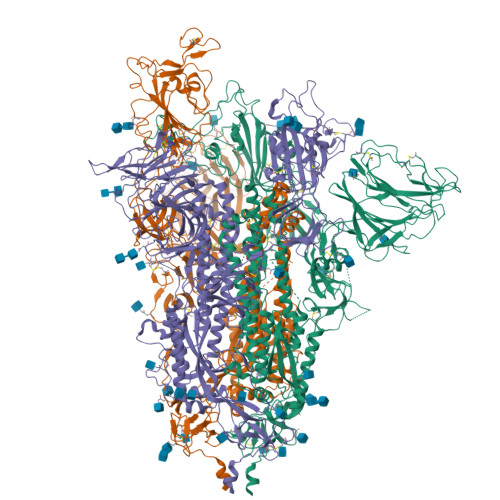
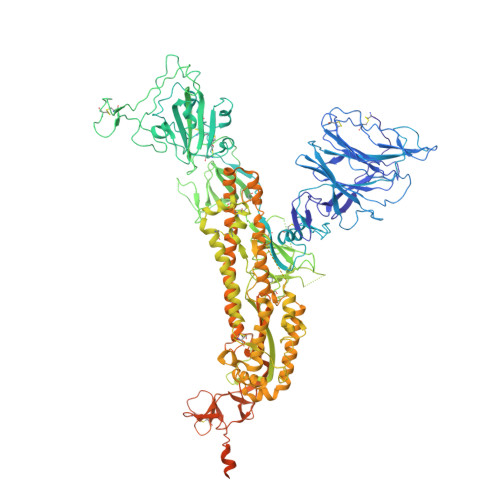
The S:A222V point mutation, within the G clade, was characteristic of the 20E (EU1) SARS-CoV-2 variant identified in Spain in early summer 2020. This mutation has since reappeared in the Delta subvariant AY.4.2, raising questions about its specific effect on viral infection. We report combined serological, functional, structural and computational studies characterizing the impact of this mutation. Our results reveal that S:A222V promotes an increased RBD opening and slightly increases ACE2 binding as compared to the parent S:D614G clade. Finally, S:A222V does not reduce sera neutralization capacity, suggesting it does not affect vaccine effectiveness.
Organizational Affiliation:
Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC), Madrid, Spain.