Highly protective antimalarial antibodies via precision library generation and yeast display screening.
Banach, B.B., Tripathi, P., Da Silva Pereira, L., Gorman, J., Nguyen, T.D., Dillon, M., Fahad, A.S., Kiyuka, P.K., Madan, B., Wolfe, J.R., Bonilla, B., Flynn, B., Francica, J.R., Hurlburt, N.K., Kisalu, N.K., Liu, T., Ou, L., Rawi, R., Schon, A., Shen, C.H., Teng, I.T., Zhang, B., Pancera, M., Idris, A.H., Seder, R.A., Kwong, P.D., DeKosky, B.J.(2022) J Exp Med 219
- PubMed: 35736810
- DOI: https://doi.org/10.1084/jem.20220323
- Primary Citation of Related Structures:
7SG5, 7SG6 - PubMed Abstract:
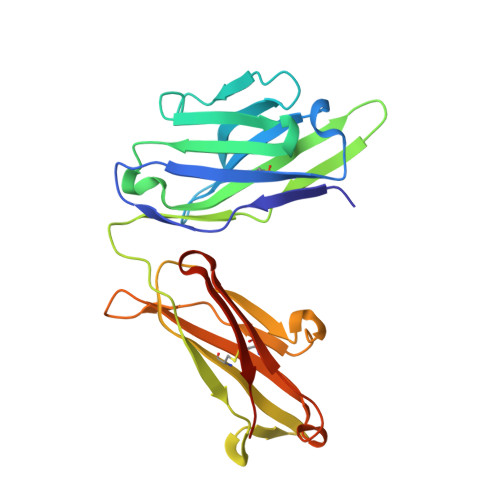
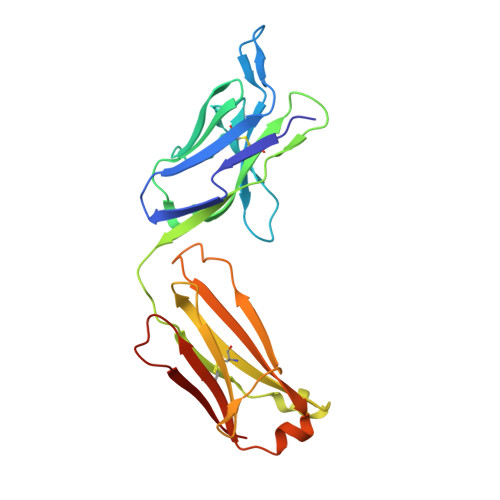
The monoclonal antibody CIS43 targets the Plasmodium falciparum circumsporozoite protein (PfCSP) and prevents malaria infection in humans for up to 9 mo following a single intravenous administration. To enhance the potency and clinical utility of CIS43, we used iterative site-saturation mutagenesis and DNA shuffling to screen precise gene-variant yeast display libraries for improved PfCSP antigen recognition. We identified several mutations that improved recognition, predominately in framework regions, and combined these to produce a panel of antibody variants. The most improved antibody, CIS43_Var10, had three mutations and showed approximately sixfold enhanced protective potency in vivo compared to CIS43. Co-crystal and cryo-electron microscopy structures of CIS43_Var10 with the peptide epitope or with PfCSP, respectively, revealed functional roles for each of these mutations. The unbiased site-directed mutagenesis and screening pipeline described here represent a powerful approach to enhance protective potency and to enable broader clinical use of antimalarial antibodies.
Organizational Affiliation:
Bioengineering Graduate Program, The University of Kansas, Lawrence, KS.