Insights Into Drug Repurposing, as Well as Specificity and Compound Properties of Piperidine-Based SARS-CoV-2 PLpro Inhibitors.
Calleja, D.J., Kuchel, N., Lu, B.G.C., Birkinshaw, R.W., Klemm, T., Doerflinger, M., Cooney, J.P., Mackiewicz, L., Au, A.E., Yap, Y.Q., Blackmore, T.R., Katneni, K., Crighton, E., Newman, J., Jarman, K.E., Call, M.J., Lechtenberg, B.C., Czabotar, P.E., Pellegrini, M., Charman, S.A., Lowes, K.N., Mitchell, J.P., Nachbur, U., Lessene, G., Komander, D.(2022) Front Chem 10: 861209-861209
- PubMed: 35494659
- DOI: https://doi.org/10.3389/fchem.2022.861209
- Primary Citation of Related Structures:
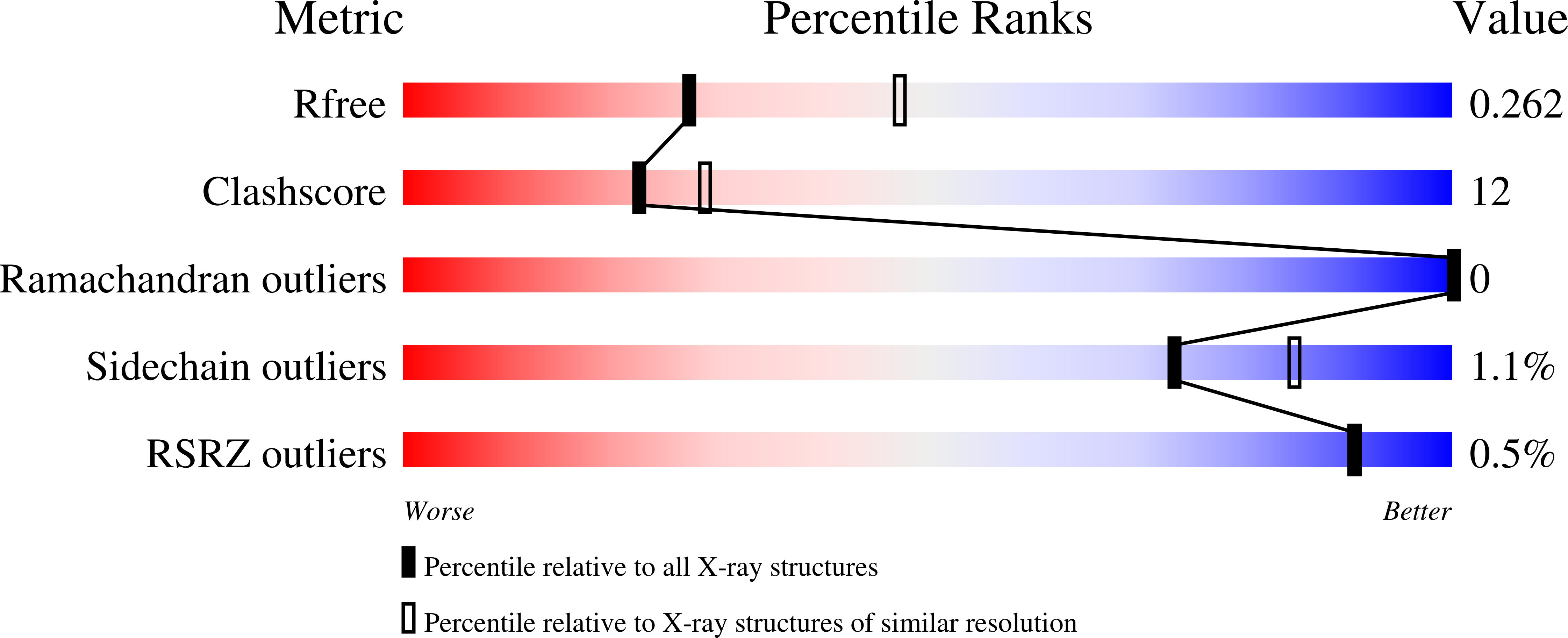
7TZJ - PubMed Abstract:
The COVID-19 pandemic continues unabated, emphasizing the need for additional antiviral treatment options to prevent hospitalization and death of patients infected with SARS-CoV-2. The papain-like protease (PLpro) domain is part of the SARS-CoV-2 non-structural protein (nsp)-3, and represents an essential protease and validated drug target for preventing viral replication. PLpro moonlights as a deubiquitinating (DUB) and deISGylating enzyme, enabling adaptation of a DUB high throughput (HTS) screen to identify PLpro inhibitors. Drug repurposing has been a major focus through the COVID-19 pandemic as it may provide a fast and efficient route for identifying clinic-ready, safe-in-human antivirals. We here report our effort to identify PLpro inhibitors by screening the ReFRAME library of 11,804 compounds, showing that none inhibit PLpro with any reasonable activity or specificity to justify further progression towards the clinic. We also report our latest efforts to improve piperidine-scaffold inhibitors, 5c and 3k , originally developed for SARS-CoV PLpro. We report molecular details of binding and selectivity, as well as in vitro absorption, distribution, metabolism and excretion (ADME) studies of this scaffold. A co-crystal structure of SARS-CoV-2 PLpro bound to inhibitor 3k guides medicinal chemistry efforts to improve binding and ADME characteristics. We arrive at compounds with improved and favorable solubility and stability characteristics that are tested for inhibiting viral replication. Whilst still requiring significant improvement, our optimized small molecule inhibitors of PLpro display decent antiviral activity in an in vitro SARS-CoV-2 infection model, justifying further optimization.
Organizational Affiliation:
Department of Medical Biology, Walter and Eliza Hall Institute, University of Melbourne, Melbourne, VIC, Australia.