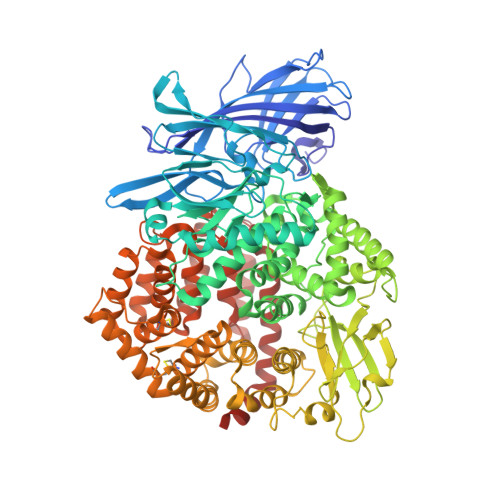
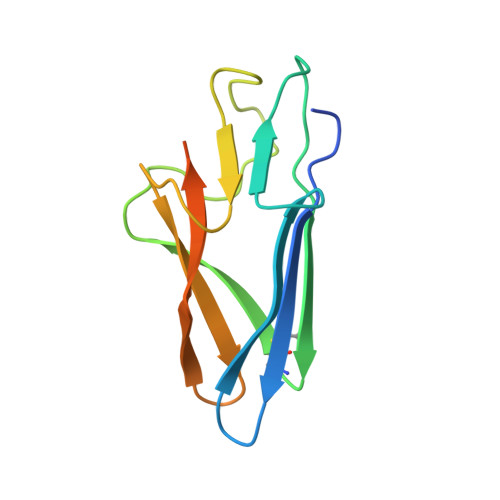
Structures of a deltacoronavirus spike protein bound to porcine and human receptors.
Ji, W., Peng, Q., Fang, X., Li, Z., Li, Y., Xu, C., Zhao, S., Li, J., Chen, R., Mo, G., Wei, Z., Xu, Y., Li, B., Zhang, S.(2022) Nat Commun 13: 1467-1467
- PubMed: 35304871
- DOI: https://doi.org/10.1038/s41467-022-29062-5
- Primary Citation of Related Structures:
7VPP, 7VPQ - PubMed Abstract:
Porcine deltacoronavirus (PDCoV) can experimentally infect a variety of animals. Human infection by PDCoV has also been reported. Consistently, PDCoV can use aminopeptidase N (APN) from different host species as receptors to enter cells. To understand this broad receptor usage and interspecies transmission of PDCoV, we determined the crystal structures of the receptor binding domain (RBD) of PDCoV spike protein bound to human APN (hAPN) and porcine APN (pAPN), respectively. The structures of the two complexes exhibit high similarity. PDCoV RBD binds to common regions on hAPN and pAPN, which are different from the sites engaged by two alphacoronaviruses: HCoV-229E and porcine respiratory coronavirus (PRCoV). Based on structure guided mutagenesis, we identified conserved residues on hAPN and pAPN that are essential for PDCoV binding and infection. We report the detailed mechanism for how a deltacoronavirus recognizes homologous receptors and provide insights into the cross-species transmission of PDCoV.
Organizational Affiliation:
College of Life Sciences, Nanjing Agricultural University, Nanjing, 210095, China.