De novo design of protein interactions with learned surface fingerprints.
Gainza, P., Wehrle, S., Van Hall-Beauvais, A., Marchand, A., Scheck, A., Harteveld, Z., Buckley, S., Ni, D., Tan, S., Sverrisson, F., Goverde, C., Turelli, P., Raclot, C., Teslenko, A., Pacesa, M., Rosset, S., Georgeon, S., Marsden, J., Petruzzella, A., Liu, K., Xu, Z., Chai, Y., Han, P., Gao, G.F., Oricchio, E., Fierz, B., Trono, D., Stahlberg, H., Bronstein, M., Correia, B.E.(2023) Nature 617: 176-184
- PubMed: 37100904
- DOI: https://doi.org/10.1038/s41586-023-05993-x
- Primary Citation of Related Structures:
7XAD, 7XYQ, 7ZRV, 7ZSD, 7ZSS - PubMed Abstract:
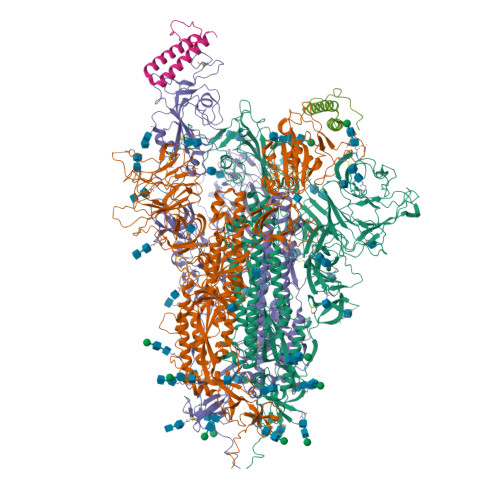
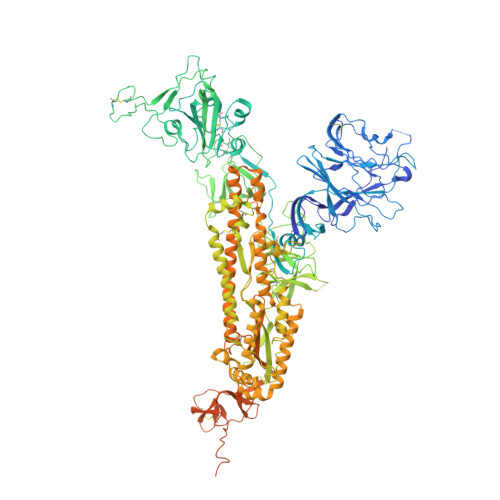
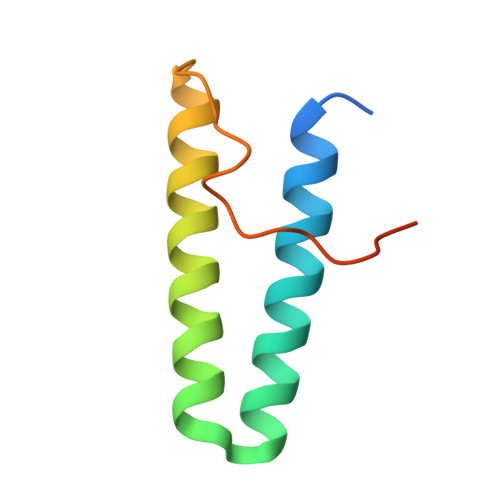
Physical interactions between proteins are essential for most biological processes governing life 1 . However, the molecular determinants of such interactions have been challenging to understand, even as genomic, proteomic and structural data increase. This knowledge gap has been a major obstacle for the comprehensive understanding of cellular protein-protein interaction networks and for the de novo design of protein binders that are crucial for synthetic biology and translational applications 2-9 . Here we use a geometric deep-learning framework operating on protein surfaces that generates fingerprints to describe geometric and chemical features that are critical to drive protein-protein interactions 10 . We hypothesized that these fingerprints capture the key aspects of molecular recognition that represent a new paradigm in the computational design of novel protein interactions. As a proof of principle, we computationally designed several de novo protein binders to engage four protein targets: SARS-CoV-2 spike, PD-1, PD-L1 and CTLA-4. Several designs were experimentally optimized, whereas others were generated purely in silico, reaching nanomolar affinity with structural and mutational characterization showing highly accurate predictions. Overall, our surface-centric approach captures the physical and chemical determinants of molecular recognition, enabling an approach for the de novo design of protein interactions and, more broadly, of artificial proteins with function.
Organizational Affiliation:
Laboratory of Protein Design and Immunoengineering, Institute of Bioengineering, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland.