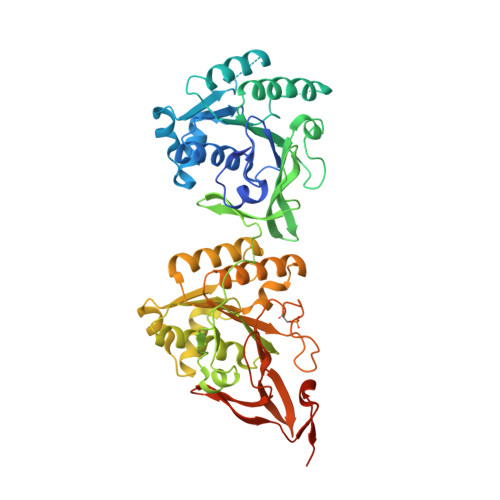
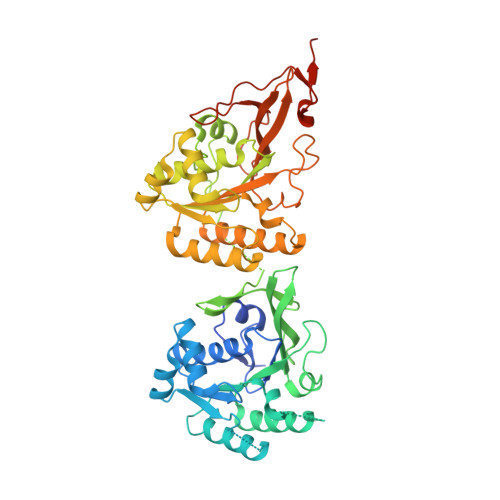
Elucidation of master allostery essential for circadian clock oscillation in cyanobacteria.
Furuike, Y., Mukaiyama, A., Ouyang, D., Ito-Miwa, K., Simon, D., Yamashita, E., Kondo, T., Akiyama, S.(2022) Sci Adv 8: eabm8990-eabm8990
- PubMed: 35427168
- DOI: https://doi.org/10.1126/sciadv.abm8990
- Primary Citation of Related Structures:
7DXQ, 7DY2, 7DYI, 7DYJ, 7DYK, 7V3X - PubMed Abstract:
Spatiotemporal allostery is the source of complex but ordered biological phenomena. To identify the structural basis for allostery that drives the cyanobacterial circadian clock, we crystallized the clock protein KaiC in four distinct states, which cover a whole cycle of phosphor-transfer events at Ser 431 and Thr 432 . The minimal set of allosteric events required for oscillatory nature is a bidirectional coupling between the coil-to-helix transition of the Ser 431 -dependent phospho-switch in the C-terminal domain of KaiC and adenosine 5'-diphosphate release from its N-terminal domain during adenosine triphosphatase cycle. An engineered KaiC protein oscillator consisting of a minimal set of the identified master allosteric events exhibited a monophosphorylation cycle of Ser 431 with a temperature-compensated circadian period, providing design principles for simple posttranslational biochemical circadian oscillators.
Organizational Affiliation:
Research Center of Integrative Molecular Systems (CIMoS), Institute for Molecular Science, National Institutes of Natural Sciences, 38 Nishigo-Naka, Myodaiji, Okazaki 444-8585, Japan.