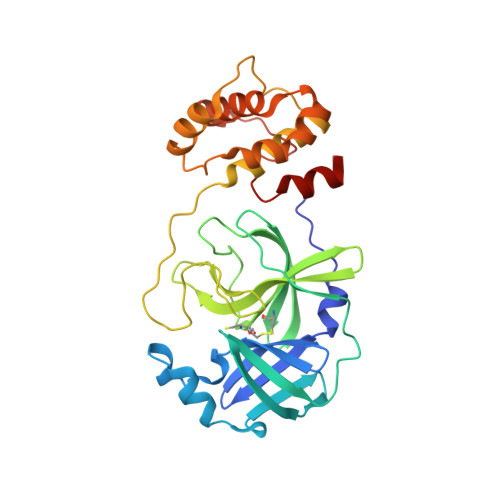
SARS-CoV-2 M pro responds to oxidation by forming disulfide and NOS/SONOS bonds.
Reinke, P.Y.A., Schubert, R., Oberthur, D., Galchenkova, M., Rahmani Mashhour, A., Gunther, S., Chretien, A., Round, A., Seychell, B.C., Norton-Baker, B., Kim, C., Schmidt, C., Koua, F.H.M., Tolstikova, A., Ewert, W., Pena Murillo, G.E., Mills, G., Kirkwood, H., Brognaro, H., Han, H., Koliyadu, J., Schulz, J., Bielecki, J., Lieske, J., Maracke, J., Knoska, J., Lorenzen, K., Brings, L., Sikorski, M., Kloos, M., Vakili, M., Vagovic, P., Middendorf, P., de Wijn, R., Bean, R., Letrun, R., Han, S., Falke, S., Geng, T., Sato, T., Srinivasan, V., Kim, Y., Yefanov, O.M., Gelisio, L., Beck, T., Dore, A.S., Mancuso, A.P., Betzel, C., Bajt, S., Redecke, L., Chapman, H.N., Meents, A., Turk, D., Hinrichs, W., Lane, T.J.(2024) Nat Commun 15: 3827-3827
- PubMed: 38714735
- DOI: https://doi.org/10.1038/s41467-024-48109-3
- Primary Citation of Related Structures:
7PXZ, 7PZQ, 7Z2K - PubMed Abstract:
The main protease (M pro ) of SARS-CoV-2 is critical for viral function and a key drug target. M pro is only active when reduced; turnover ceases upon oxidation but is restored by re-reduction. This suggests the system has evolved to survive periods in an oxidative environment, but the mechanism of this protection has not been confirmed. Here, we report a crystal structure of oxidized M pro showing a disulfide bond between the active site cysteine, C145, and a distal cysteine, C117. Previous work proposed this disulfide provides the mechanism of protection from irreversible oxidation. M pro forms an obligate homodimer, and the C117-C145 structure shows disruption of interactions bridging the dimer interface, implying a correlation between oxidation and dimerization. We confirm dimer stability is weakened in solution upon oxidation. Finally, we observe the protein's crystallization behavior is linked to its redox state. Oxidized M pro spontaneously forms a distinct, more loosely packed lattice. Seeding with crystals of this lattice yields a structure with an oxidation pattern incorporating one cysteine-lysine-cysteine (SONOS) and two lysine-cysteine (NOS) bridges. These structures further our understanding of the oxidative regulation of M pro and the crystallization conditions necessary to study this structurally.
Organizational Affiliation:
Center for Free-Electron Laser Science CFEL, Deutsches Elektronen-Synchrotron DESY, Notkestr. 85, 22607, Hamburg, Germany.