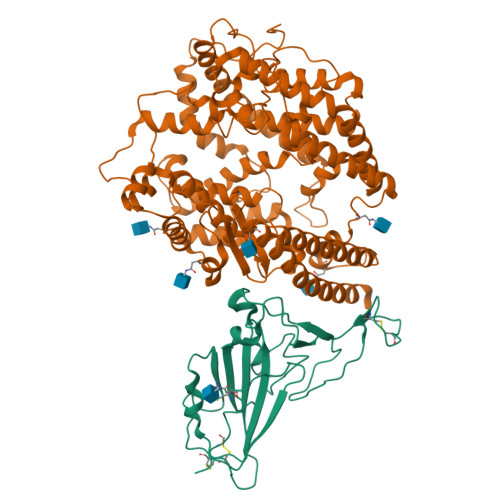
Cryo-EM structures and binding of mouse and human ACE2 to SARS-CoV-2 variants of concern indicate that mutations enabling immune escape could expand host range.
Ni, D., Turelli, P., Beckert, B., Nazarov, S., Uchikawa, E., Myasnikov, A., Pojer, F., Trono, D., Stahlberg, H., Lau, K.(2023) PLoS Pathog 19: e1011206-e1011206
- PubMed: 37018380
- DOI: https://doi.org/10.1371/journal.ppat.1011206
- Primary Citation of Related Structures:
8AQS, 8AQT, 8AQU, 8AQV, 8AQW - PubMed Abstract:
Investigation of potential hosts of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is crucial to understanding future risks of spillover and spillback. SARS-CoV-2 has been reported to be transmitted from humans to various animals after requiring relatively few mutations. There is significant interest in describing how the virus interacts with mice as they are well adapted to human environments, are used widely as infection models and can be infected. Structural and binding data of the mouse ACE2 receptor with the Spike protein of newly identified SARS-CoV-2 variants are needed to better understand the impact of immune system evading mutations present in variants of concern (VOC). Previous studies have developed mouse-adapted variants and identified residues critical for binding to heterologous ACE2 receptors. Here we report the cryo-EM structures of mouse ACE2 bound to trimeric Spike ectodomains of four different VOC: Beta, Omicron BA.1, Omicron BA.2.12.1 and Omicron BA.4/5. These variants represent the oldest to the newest variants known to bind the mouse ACE2 receptor. Our high-resolution structural data complemented with bio-layer interferometry (BLI) binding assays reveal a requirement for a combination of mutations in the Spike protein that enable binding to the mouse ACE2 receptor.
Organizational Affiliation:
Laboratory of Biological Electron Microscopy (LBEM), Institute of Physics, School of Basic Science, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland.