AI-Accelerated Design of Targeted Covalent Inhibitors for SARS-CoV-2.
Joshi, R.P., Schultz, K.J., Wilson, J.W., Kruel, A., Varikoti, R.A., Kombala, C.J., Kneller, D.W., Galanie, S., Phillips, G., Zhang, Q., Coates, L., Parvathareddy, J., Surendranathan, S., Kong, Y., Clyde, A., Ramanathan, A., Jonsson, C.B., Brandvold, K.R., Zhou, M., Head, M.S., Kovalevsky, A., Kumar, N.(2023) J Chem Inf Model 63: 1438-1453
- PubMed: 36808989
- DOI: https://doi.org/10.1021/acs.jcim.2c01377
- Primary Citation of Related Structures:
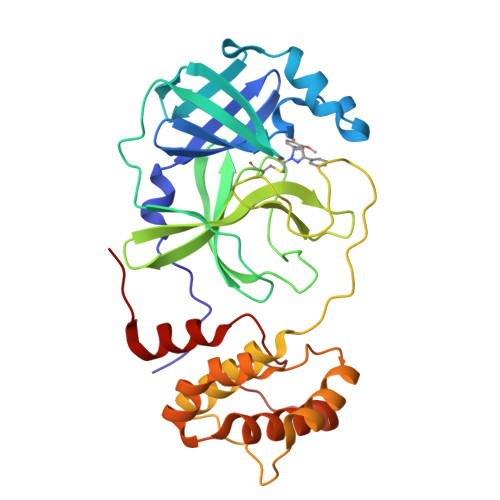
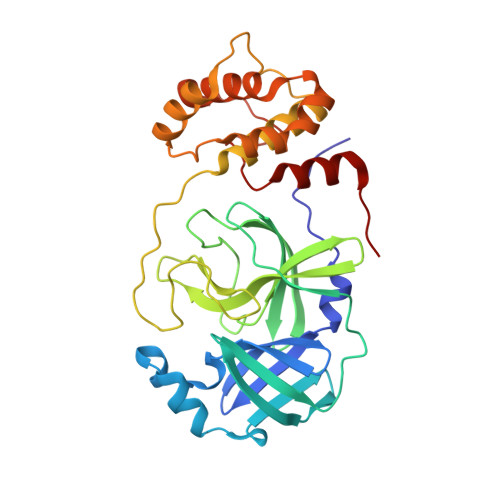
8DL9, 8DLB, 8DMD - PubMed Abstract:
Direct-acting antivirals for the treatment of the COVID-19 pandemic caused by the SARS-CoV-2 virus are needed to complement vaccination efforts. Given the ongoing emergence of new variants, automated experimentation, and active learning based fast workflows for antiviral lead discovery remain critical to our ability to address the pandemic's evolution in a timely manner. While several such pipelines have been introduced to discover candidates with noncovalent interactions with the main protease (M pro ), here we developed a closed-loop artificial intelligence pipeline to design electrophilic warhead-based covalent candidates. This work introduces a deep learning-assisted automated computational workflow to introduce linkers and an electrophilic "warhead" to design covalent candidates and incorporates cutting-edge experimental techniques for validation. Using this process, promising candidates in the library were screened, and several potential hits were identified and tested experimentally using native mass spectrometry and fluorescence resonance energy transfer (FRET)-based screening assays. We identified four chloroacetamide-based covalent inhibitors of M pro with micromolar affinities (K I of 5.27 μM) using our pipeline. Experimentally resolved binding modes for each compound were determined using room-temperature X-ray crystallography, which is consistent with the predicted poses. The induced conformational changes based on molecular dynamics simulations further suggest that the dynamics may be an important factor to further improve selectivity, thereby effectively lowering K I and reducing toxicity. These results demonstrate the utility of our modular and data-driven approach for potent and selective covalent inhibitor discovery and provide a platform to apply it to other emerging targets.
Organizational Affiliation:
Earth and Biological Sciences Directorate, Pacific Northwest National Laboratory, Richland, Washington 99352, United States.