Structural Insights of the SARS-CoV-2 Nucleocapsid Protein: Implications for the Inner-workings of Rapid Antigen Tests.
Casasanta, M.A., Jonaid, G.M., Kaylor, L., Luqiu, W.Y., DiCecco, L.A., Solares, M.J., Berry, S., Dearnaley, W.J., Kelly, D.F.(2023) Microsc Microanal 29: 649-657
- PubMed: 37749713
- DOI: https://doi.org/10.1093/micmic/ozac036
- Primary Citation of Related Structures:
8FD5, 8FG2 - PubMed Abstract:
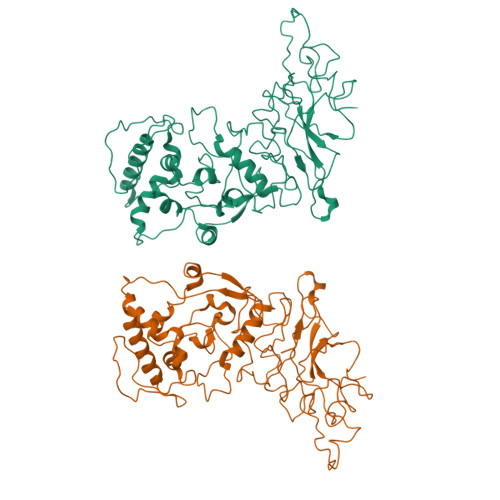
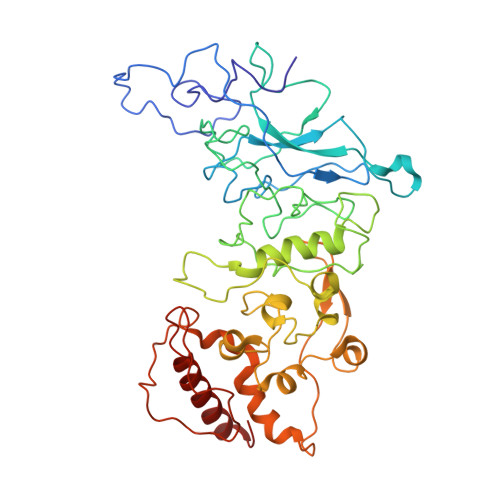
The nucleocapsid (N) protein is an abundant component of SARS-CoV-2 and a key analyte for lateral-flow rapid antigen tests. Here, we present new structural insights for the SARS-CoV-2 N protein using cryo-electron microscopy (EM) and molecular modeling tools. Epitope mapping based on structural data supported host-immune interactions in the C-terminal portion of the protein, while other regions revealed protein-protein interaction sites. Complementary modeling results suggested that N protein structures from known variants of concern (VOC) are nearly 100% conserved at specific antibody-binding sites. Collectively, these results suggest that rapid tests that target the nucleocapsid C-terminal domain should have similar accuracy across all VOCs. In addition, our combined structural modeling workflow may guide the design of immune therapies to counter viral processes as we plan for future variants and pandemics.
Organizational Affiliation:
Department of Biomedical Engineering, Pennsylvania State University, University Park, PA 16802, USA.