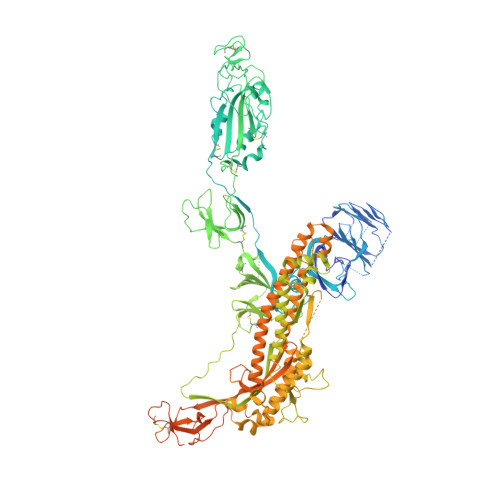
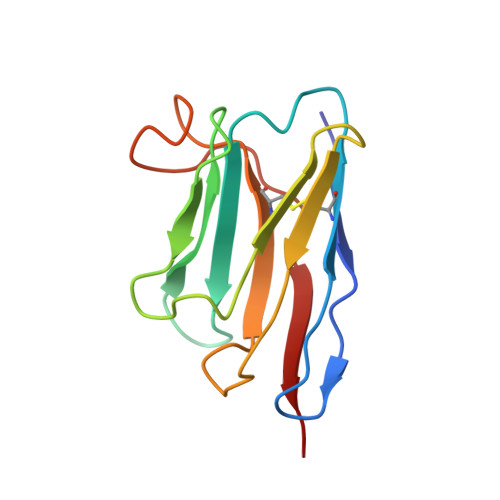
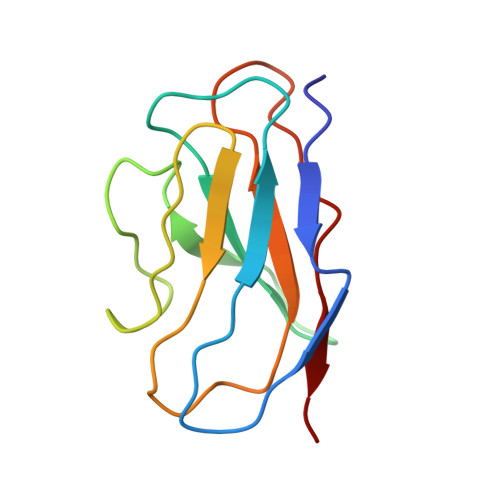
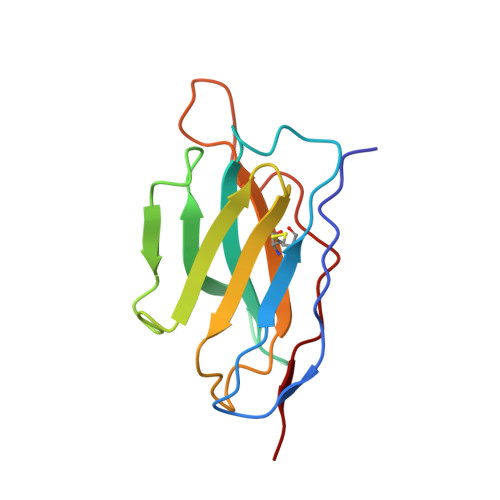
Inactivated vaccine-elicited potent antibodies can broadly neutralize SARS-CoV-2 circulating variants.
Liu, Y., Wang, Z., Zhuang, X., Zhang, S., Chen, Z., Zou, Y., Sheng, J., Li, T., Tai, W., Yu, J., Wang, Y., Zhang, Z., Chen, Y., Tong, L., Yu, X., Wu, L., Chen, D., Zhang, R., Jin, N., Shen, W., Zhao, J., Tian, M., Wang, X., Cheng, G.(2023) Nat Commun 14: 2179-2179
- PubMed: 37069158
- DOI: https://doi.org/10.1038/s41467-023-37926-7
- Primary Citation of Related Structures:
7X2H, 7XD2, 8H07, 8H08 - PubMed Abstract:
A full understanding of the inactivated COVID-19 vaccine-mediated antibody responses to SARS-CoV-2 circulating variants will inform vaccine effectiveness and vaccination development strategies. Here, we offer insights into the inactivated vaccine-induced antibody responses after prime-boost vaccination at both the polyclonal and monoclonal levels. We characterized the VDJ sequence of 118 monoclonal antibodies (mAbs) and found that 20 neutralizing mAbs showed varied potency and breadth against a range of variants including XBB.1.5, BQ.1.1, and BN.1. Bispecific antibodies (bsAbs) based on nonoverlapping mAbs exhibited enhanced neutralizing potency and breadth against the most antibody-evasive strains, such as XBB.1.5, BQ.1.1, and BN.1. The passive transfer of mAbs or their bsAb effectively protected female hACE2 transgenic mice from challenge with an infectious Delta or Omicron BA.2 variant. The neutralization mechanisms of these antibodies were determined by structural characterization. Overall, a broad spectrum of potent and distinct neutralizing antibodies can be induced in individuals immunized with the SARS-CoV-2 inactivated vaccine BBIBP-CorV, suggesting the application potential of inactivated vaccines and these antibodies for preventing infection by SARS-CoV-2 circulating variants.
Organizational Affiliation:
Tsinghua-Peking Joint Center for Life Sciences, School of Medicine, Tsinghua University, Beijing, 100084, China.