Molecular insights into the catalytic mechanism of plasticizer degradation by a monoalkyl phthalate hydrolase.
Chen, Y., Wang, Y., Xu, Y., Sun, J., Yang, L., Feng, C., Wang, J., Zhou, Y., Zhang, Z.M., Wang, Y.(2023) Commun Chem 6: 45-45
- PubMed: 36859434
- DOI: https://doi.org/10.1038/s42004-023-00846-0
- Primary Citation of Related Structures:
8HGV, 8HGW - PubMed Abstract:
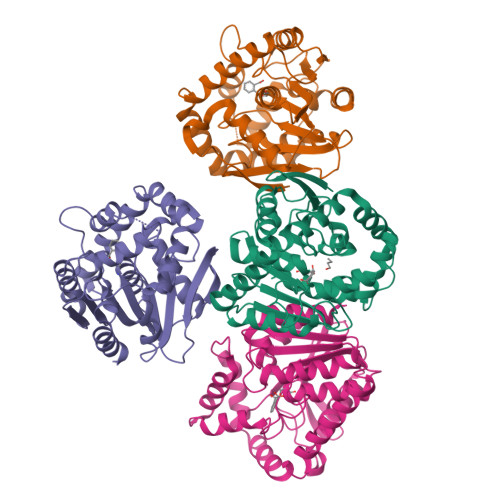
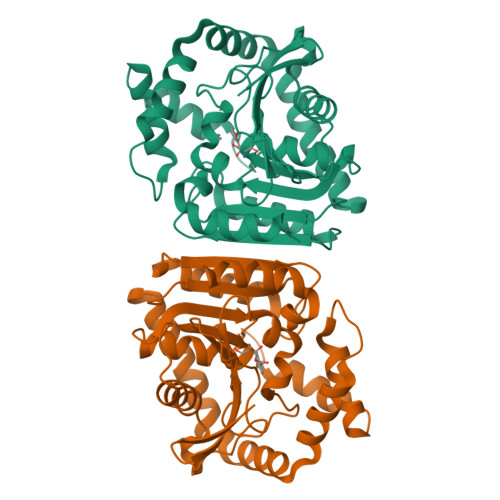
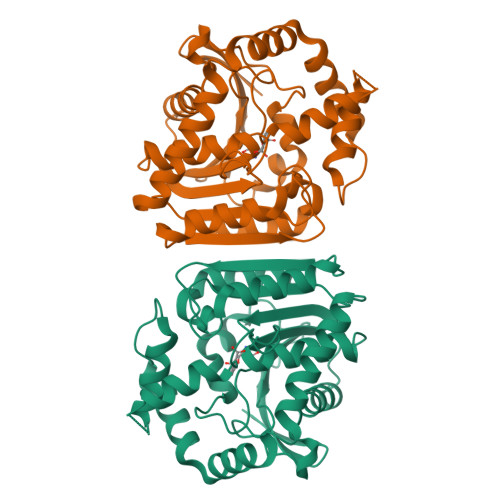
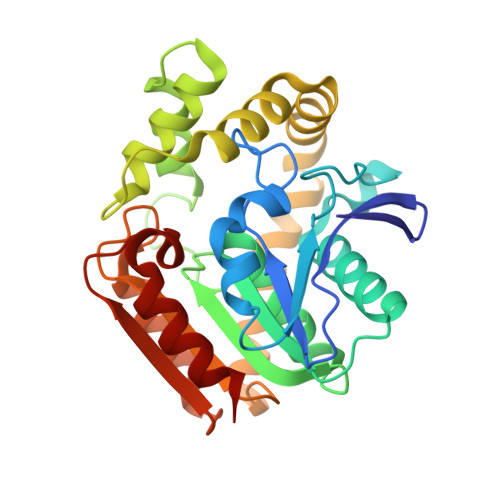
Phthalate acid esters (PAEs), a group of xenobiotic compounds used extensively as plasticizers, have attracted increasing concern for adverse effects to human health and the environment. Microbial degradation relying on PAE hydrolases is a promising treatment. However, only a limited number of PAE hydrolases were characterized to date. Here we report the structures of MehpH, a monoalkyl phthalate (MBP) hydrolase that catalyzes the reaction of MBP to phthalic acid and the corresponding alcohol, in apo and ligand-bound form. The structures reveal a positively-charged catalytic center, complementary to the negatively-charged carboxyl group on MBP, and a penetrating tunnel that serves as exit of alcohol. The study provides a first glimpse into the enzyme-substrate binding model for PAE hydrolases, leading strong support to the development of better enzymes in the future.
Organizational Affiliation:
School of Biology and Biological Engineering, South China University of Technology, Guangzhou, 510006, China.