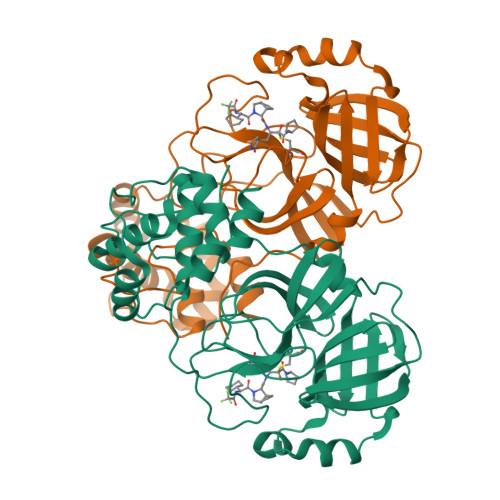
Preclinical evaluation of the SARS-CoV-2 M pro inhibitor RAY1216 shows improved pharmacokinetics compared with nirmatrelvir.
Chen, X., Huang, X., Ma, Q., Kuzmic, P., Zhou, B., Zhang, S., Chen, J., Xu, J., Liu, B., Jiang, H., Zhang, W., Yang, C., Wu, S., Huang, J., Li, H., Long, C., Zhao, X., Xu, H., Sheng, Y., Guo, Y., Niu, C., Xue, L., Xu, Y., Liu, J., Zhang, T., Spencer, J., Zhu, Z., Deng, W., Chen, X., Chen, S.H., Zhong, N., Xiong, X., Yang, Z.(2024) Nat Microbiol 9: 1075-1088
- PubMed: 38553607
- DOI: https://doi.org/10.1038/s41564-024-01618-9
- Primary Citation of Related Structures:
8IGN, 8IGO - PubMed Abstract:
Although vaccines are available for SARS-CoV-2, antiviral drugs such as nirmatrelvir are still needed, particularly for individuals in whom vaccines are less effective, such as the immunocompromised, to prevent severe COVID-19. Here we report an α-ketoamide-based peptidomimetic inhibitor of the SARS-CoV-2 main protease (M pro ), designated RAY1216. Enzyme inhibition kinetic analysis shows that RAY1216 has an inhibition constant of 8.4 nM and suggests that it dissociates about 12 times slower from M pro compared with nirmatrelvir. The crystal structure of the SARS-CoV-2 M pro :RAY1216 complex shows that RAY1216 covalently binds to the catalytic Cys145 through the α-ketoamide group. In vitro and using human ACE2 transgenic mouse models, RAY1216 shows antiviral activities against SARS-CoV-2 variants comparable to those of nirmatrelvir. It also shows improved pharmacokinetics in mice and rats, suggesting that RAY1216 could be used without ritonavir, which is co-administered with nirmatrelvir. RAY1216 has been approved as a single-component drug named 'leritrelvir' for COVID-19 treatment in China.
Organizational Affiliation:
School of Pharmaceutical Sciences (Shenzhen), Sun Yat-sen University, Shenzhen, China.