structure of ion channel
Chen, H.W., Chen, H.W.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ion channel,Voltage dependent ion channel,Green fluorescent protein (Fragment),Voltage dependent ion channel,Green fluorescent protein (Fragment),Ion transport domain-containing protein | 289 | Homo sapiens, Aequorea victoria | Mutation(s): 0 Membrane Entity: Yes | 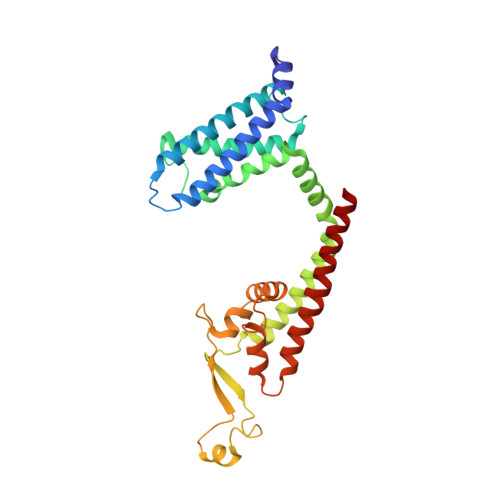 | |
UniProt | |||||
Find proteins for R1EKX3 (Emiliania huxleyi) Explore R1EKX3 Go to UniProtKB: R1EKX3 | |||||
Find proteins for R1FVI4 (Emiliania huxleyi) Explore R1FVI4 Go to UniProtKB: R1FVI4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | R1EKX3R1FVI4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ILE-ALA-ALA-ILE-HIS-ASN-ALA-ARG-ARG-LYS-LYS-ARG-GLU-ALA-ALA-ALA-ALA-HIS-LYS-ALA | 20 | Homo sapiens | Mutation(s): 0 Membrane Entity: Yes | 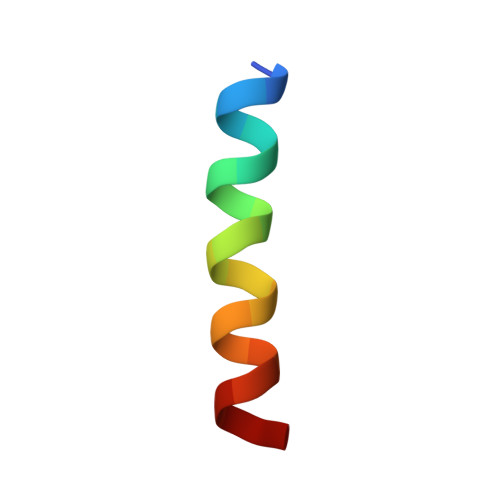 | |
UniProt | |||||
Find proteins for R1DBK9 (Emiliania huxleyi) Explore R1DBK9 Go to UniProtKB: R1DBK9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | R1DBK9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| POV Query on POV | G [auth A], L [auth B], R [auth C], V [auth D] | (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate C42 H82 N O8 P WTJKGGKOPKCXLL-PFDVCBLKSA-N |  | ||
| CLR Query on CLR | H [auth A] I [auth A] J [auth A] M [auth B] N [auth B] | CHOLESTEROL C27 H46 O HVYWMOMLDIMFJA-DPAQBDIFSA-N |  | ||
| CA (Subject of Investigation/LOI) Query on CA | F [auth A], K [auth B], P [auth C], Q [auth C] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 31971134 |