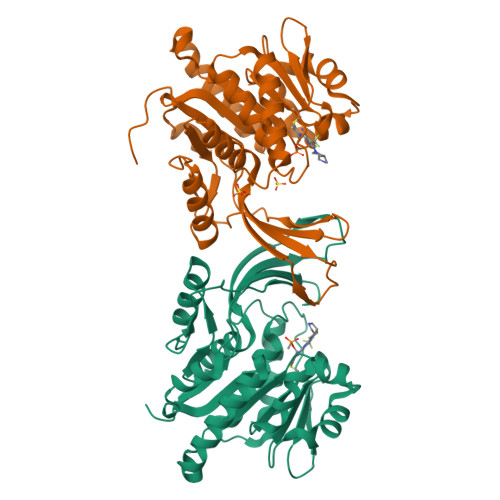
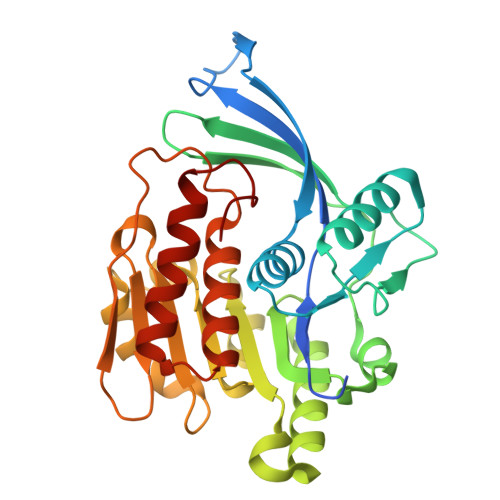
Discovery of BI-9787, a potent zwitterionic ketohexokinase inhibitor with oral bioavailability.
Heine, N., Weber, A., Pautsch, A., Gottschling, D., Uphues, I., Bauer, M., Ebenhoch, R., Magarkar, A., Nosse, B., Kley, J.T.(2024) Bioorg Med Chem Lett 112: 129930-129930
- PubMed: 39179180
- DOI: https://doi.org/10.1016/j.bmcl.2024.129930
- Primary Citation of Related Structures:
8OMJ, 8OMK, 9FHD, 9FHE - PubMed Abstract:
Fructose metabolism by ketohexokinase (KHK) is implicated in a variety of metabolic disorders. KHK inhibition is a potential therapeutic strategy for the treatment of diseases including diabetes, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis. The first small-molecule KHK-inhibitors have entered clinical trials, but it remains unclear if systemic inhibition of KHK by small-molecules will eventually benefit patients. Here we report the discovery of BI-9787, a potent, zwitterionic KHK inhibitor characterized by high permeability and favorable oral rat pharmacokinetics. BI-9787 was identified by optimizing chemical starting points generated via a ligand-based virtual screening of Boehringer's virtual library of synthetically accessible compounds (BICLAIM). It serves as a high-quality in vitro and in vivo tool compound for investigating the role of fructose metabolism in disease.
Organizational Affiliation:
Boehringer Ingelheim Pharma GmbH & Co. KG, Global Medicinal Chemistry, Birkendorfer Straße 65, 88397 Biberach an der Riß, Germany.