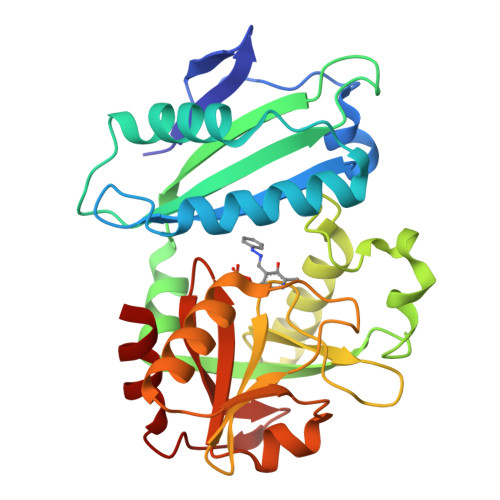
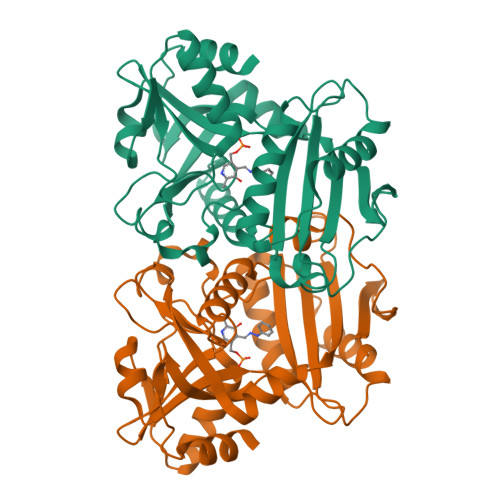
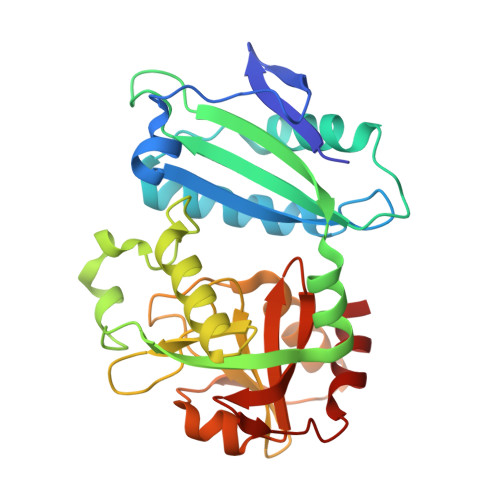
Expanded Substrate Specificity in D-Amino Acid Transaminases: A Case Study of Transaminase from Blastococcus saxobsidens.
Shilova, S.A., Matyuta, I.O., Petrova, E.S., Nikolaeva, A.Y., Rakitina, T.V., Minyaev, M.E., Boyko, K.M., Popov, V.O., Bezsudnova, E.Y.(2023) Int J Mol Sci 24
- PubMed: 38003383
- DOI: https://doi.org/10.3390/ijms242216194
- Primary Citation of Related Structures:
8PNW, 8PNY - PubMed Abstract:
Enzymes with expanded substrate specificity are good starting points for the design of biocatalysts for target reactions. However, the structural basis of the expanded substrate specificity is still elusive, especially in the superfamily of pyridoxal-5'-phosphate-dependent transaminases, which are characterized by a conserved organization of both the active site and functional dimer. Here, we analyze the structure-function relationships in a non-canonical D-amino acid transaminase from Blastococcus saxobsidens , which is active towards D-amino acids and primary ( R )-amines. A detailed study of the enzyme includes a kinetic analysis of its substrate scope and a structural analysis of the holoenzyme and its complex with phenylhydrazine-a reversible inhibitor and analogue of ( R )-1-phenylethylamine-a benchmark substrate of ( R )-selective amine transaminases. We suggest that the features of the active site of transaminase from B. saxobsidens , such as the flexibility of the R34 and R96 residues, the lack of bulky residues in the β-turn at the entrance to the active site, and the short O-pocket loop, facilitate the binding of substrates with and without α-carboxylate groups. The proposed structural determinants of the expanded substrate specificity can be used for the design of transaminases for the stereoselective amination of keto compounds.
Organizational Affiliation:
Bach Institute of Biochemistry, Research Centre of Biotechnology, Russian Academy of Sciences, Moscow 119071, Russia.