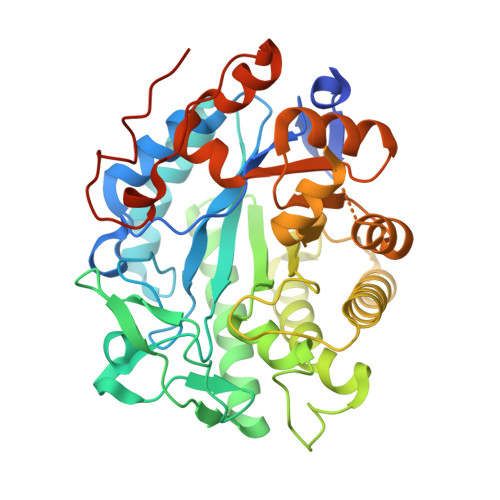
Loop 6 and the beta-hairpin flap are structural hotspots that determine cofactor specificity in the FMN-dependent family of ene-reductases.
Kerschbaumer, B., Totaro, M.G., Friess, M., Breinbauer, R., Bijelic, A., Macheroux, P.(2024) FEBS J 291: 1560-1574
- PubMed: 38263933
- DOI: https://doi.org/10.1111/febs.17055
- Primary Citation of Related Structures:
8QMX, 8QN3 - PubMed Abstract:
Flavin mononucleotide (FMN)-dependent ene-reductases constitute a large family of oxidoreductases that catalyze the enantiospecific reduction of carbon-carbon double bonds. The reducing equivalents required for substrate reduction are obtained from reduced nicotinamide by hydride transfer. Most ene-reductases significantly prefer, or exclusively accept, either NADPH or NADH. Despite their usefulness in biocatalytic applications, the structural determinants for cofactor preference remain elusive. We employed the NADPH-preferring 12-oxophytodienoic acid reductase 3 from Solanum lycopersicum (SlOPR3) as a model enzyme of the ene-reductase family and applied computational and structural methods to investigate the binding specificity of the reducing coenzymes. Initial docking results indicated that the arginine triad R283, R343, and R366 residing on and close to a critical loop at the active site (loop 6) are the main contributors to NADPH binding. In contrast, NADH binds unfavorably in the opposite direction toward the β-hairpin flap within a largely hydrophobic region. Notably, the crystal structures of SlOPR3 in complex with either NADPH 4 or NADH 4 corroborated these different binding modes. Molecular dynamics simulations confirmed NADH binding near the β-hairpin flap and provided structural explanations for the low binding affinity of NADH to SlOPR3. We postulate that cofactor specificity is determined by the arginine triad/loop 6 and the residue(s) controlling access to a hydrophobic cleft formed by the β-hairpin flap. Thus, NADPH preference depends on a properly positioned arginine triad, whereas granting access to the hydrophobic cleft at the β-hairpin flap favors NADH binding.
Organizational Affiliation:
Institute of Biochemistry, Graz University of Technology, Austria.