Molecular Mechanisms of ACTG2 Mutations Linked to Visceral Myopathy
Ceron, R.H., Baez-Cruz, F.A., Palmer, N.J., Carman, P.J., Boczkowska, M., Heuckeroth, R.O., Ostap, E.M., Dominguez, R.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin, gamma-enteric smooth muscle | 376 | Homo sapiens | Mutation(s): 1 Gene Names: ACTG2, ACTA3, ACTL3, ACTSG EC: 3.6.4 | 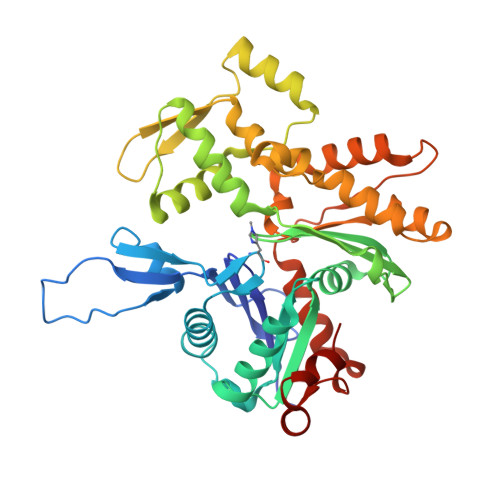 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P63267 (Homo sapiens) Explore P63267 Go to UniProtKB: P63267 | |||||
PHAROS: P63267 GTEx: ENSG00000163017 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P63267 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ADP Query on ADP | K [auth A], M [auth B], P [auth C], Q [auth D], S [auth E] | ADENOSINE-5'-DIPHOSPHATE C10 H15 N5 O10 P2 XTWYTFMLZFPYCI-KQYNXXCUSA-N |  | ||
| MG Query on MG | L [auth A], N [auth B], O [auth C], R [auth D], T [auth E] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Modified Residues 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| HIC Query on HIC | A, B, C, D, E | L-PEPTIDE LINKING | C7 H11 N3 O2 |  | HIS |
| HYP Query on HYP | F, G, H, I, J | L-PEPTIDE LINKING | C5 H9 N O3 |  | PRO |
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_002366 Query on PRD_002366 | F, G, H, I, J | Phalloidin | Peptide-like / Toxin |  | |
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC | 4.0 |
| MODEL REFINEMENT | PHENIX | 1.20.1 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease (NIH/NIDDK) | United States | -- |