Asymmetric Engagement of Dimeric CRL3KBTBD4 by the Molecular Glue UM171 Licenses Degradation of HDAC1/2 Complexes
Zheng, N., Xie, X., Liau, B.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Isoform 2 of Kelch repeat and BTB domain-containing protein 4 | 534 | Homo sapiens | Mutation(s): 0 Gene Names: KBTBD4, BKLHD4 | 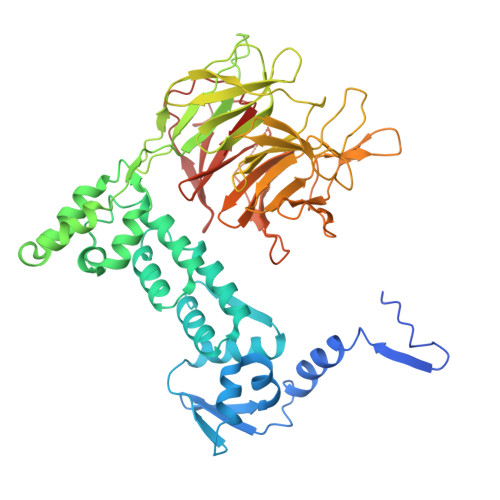 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9NVX7 (Homo sapiens) Explore Q9NVX7 Go to UniProtKB: Q9NVX7 | |||||
PHAROS: Q9NVX7 GTEx: ENSG00000123444 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9NVX7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone deacetylase 1 | 482 | Homo sapiens | Mutation(s): 0 Gene Names: HDAC1, RPD3L1 EC: 3.5.1.98 (PDB Primary Data), 3.5.1 (PDB Primary Data) | 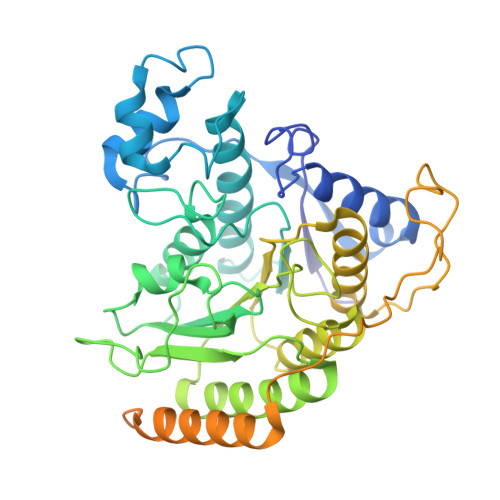 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q13547 (Homo sapiens) Explore Q13547 Go to UniProtKB: Q13547 | |||||
PHAROS: Q13547 GTEx: ENSG00000116478 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q13547 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| REST corepressor 1 | 401 | Homo sapiens | Mutation(s): 0 Gene Names: RCOR1, KIAA0071, RCOR | 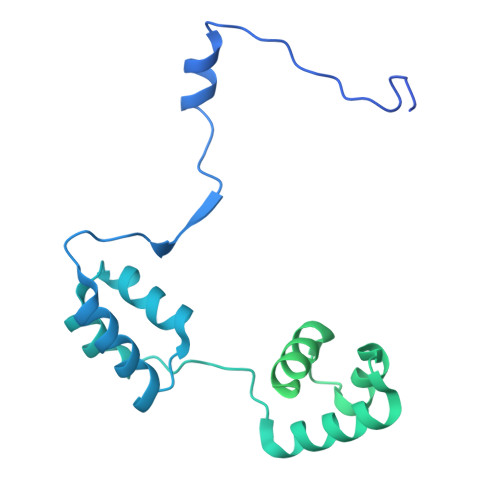 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9UKL0 (Homo sapiens) Explore Q9UKL0 Go to UniProtKB: Q9UKL0 | |||||
PHAROS: Q9UKL0 GTEx: ENSG00000089902 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9UKL0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| IHP Query on IHP | F [auth C] | INOSITOL HEXAKISPHOSPHATE C6 H18 O24 P6 IMQLKJBTEOYOSI-GPIVLXJGSA-N |  | ||
| A1ACV (Subject of Investigation/LOI) Query on A1ACV | G [auth C] | (1r,4r)-N~1~-[(7P)-2-benzyl-7-(2-methyl-2H-tetrazol-5-yl)-9H-pyrimido[4,5-b]indol-4-yl]cyclohexane-1,4-diamine C25 H27 N9 AZXXGVPWWKWGAE-IYARVYRRSA-N |  | ||
| ZN Query on ZN | E [auth C] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.21.1_5286: |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Howard Hughes Medical Institute (HHMI) | United States | -- |