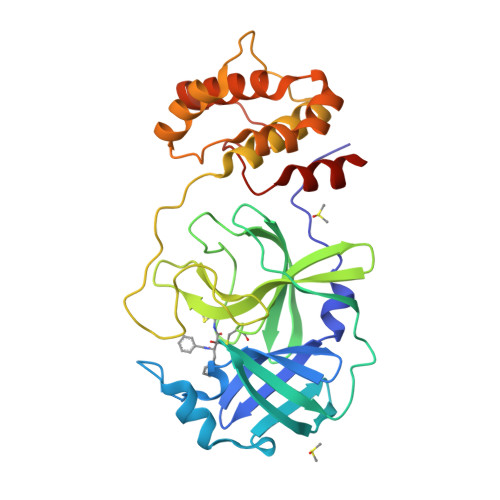
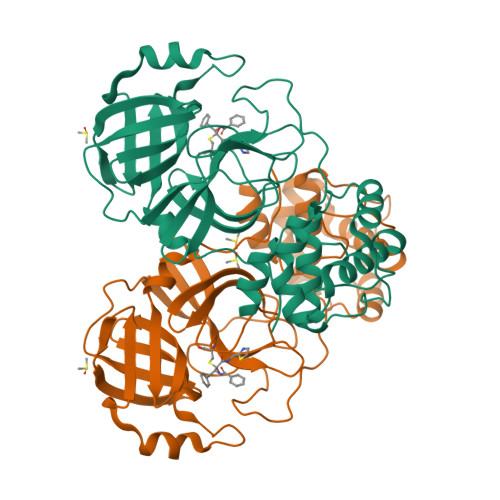
A new generation M pro inhibitor with potent activity against SARS-CoV-2 Omicron variants.
Huang, C., Shuai, H., Qiao, J., Hou, Y., Zeng, R., Xia, A., Xie, L., Fang, Z., Li, Y., Yoon, C., Huang, Q., Hu, B., You, J., Quan, B., Zhao, X., Guo, N., Zhang, S., Ma, R., Zhang, J., Wang, Y., Yang, R., Zhang, S., Nan, J., Xu, H., Wang, F., Lei, J., Chu, H., Yang, S.(2023) Signal Transduct Target Ther 8: 128-128
- PubMed: 36928316
- DOI: https://doi.org/10.1038/s41392-023-01392-w
- Primary Citation of Related Structures:
8HHT, 8HHU - PubMed Abstract:
Emerging SARS-CoV-2 variants, particularly the Omicron variant and its sublineages, continually threaten the global public health. Small molecule antivirals are an effective treatment strategy to fight against the virus. However, the first-generation antivirals either show limited clinical efficacy and/or have some defects in pharmacokinetic (PK) properties. Moreover, with increased use of these drugs across the globe, they face great pressure of drug resistance. We herein present the discovery and characterization of a new generation antiviral drug candidate (SY110), which is a potent and selective inhibitor of SARS-CoV-2 main protease (M pro ). This compound displayed potent in vitro antiviral activity against not only the predominant SARS-CoV-2 Omicron sublineage BA.5, but also other highly pathogenic human coronaviruses including SARS-CoV-1 and MERS-CoV. In the Omicron-infected K18-hACE2 mouse model, oral treatment with SY110 significantly lowered the viral burdens in lung and alleviated the virus-induced pathology. Importantly, SY110 possesses favorable PK properties with high oral drug exposure and oral bioavailability, and also an outstanding safety profile. Furthermore, SY110 exhibited sensitivity to several drug-resistance M pro mutations. Collectively, this investigation provides a promising new drug candidate against Omicron and other variants of SARS-CoV-2.
Organizational Affiliation:
State Key Laboratory of Biotherapy and Cancer Center and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, Sichuan, 610041, China.