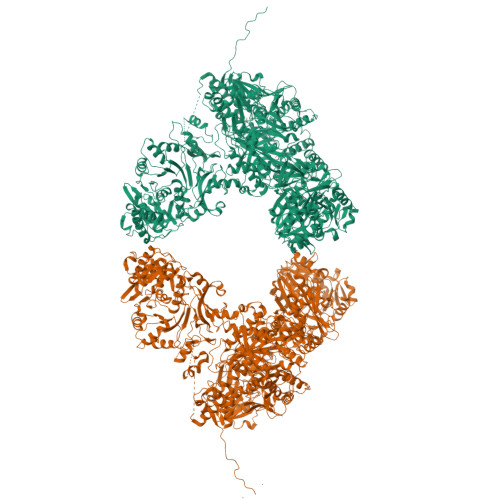
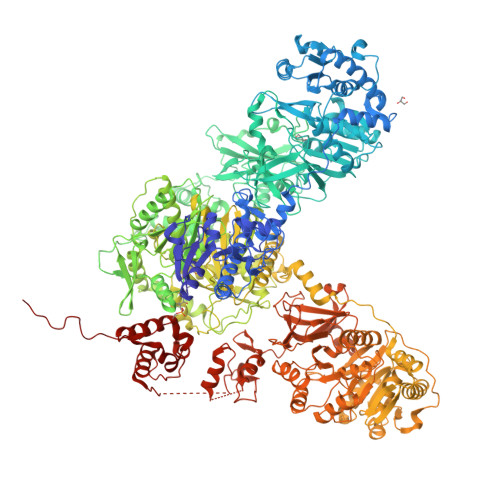
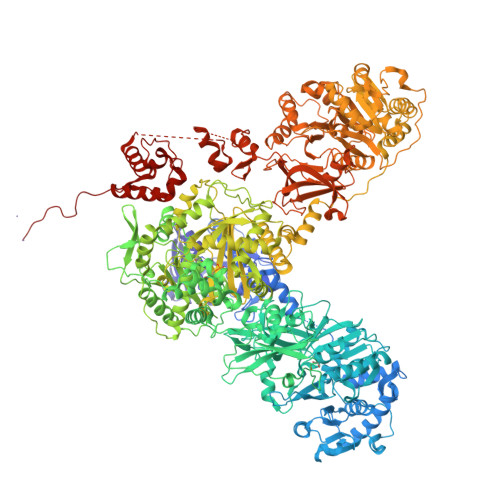
Structures and mechanism of condensation in nonribosomal peptide synthesis.
Pistofidis, A., Ma, P., Li, Z., Munro, K., Houk, K.N., Schmeing, T.M.(2024) Nature
- PubMed: 39662504
- DOI: https://doi.org/10.1038/s41586-024-08417-6
- Primary Citation of Related Structures:
9BE3, 9BE4 - PubMed Abstract:
Nonribosomal peptide synthetases (NRPSs) are mega-enzymes responsible for the biosynthesis of many clinically important natural products, from early modern medicines (penicillin, bacitracin) to current blockbuster drugs (Cubicin, vancomycin) and newly-approved therapeutics (rezafungin) 1,2 . The key chemical step in these biosyntheses is amide bond formation between aminoacyl building blocks, catalyzed by the condensation (C) domain 3 . There has been much debate over the mechanism of this reaction 3-12 . NRPS condensation has been difficult to fully characterize because it is one of many successive reactions in the NRPS synthetic cycle and because the canonical substrates are each attached transiently as thioesters to mobile carrier domains, which are often both contained in the same very flexible protein as the C domain. We have produced a dimodular NRPS protein in two parts, modified each with appropriate non-hydrolysable substrate analogs 13,14 assembled the two parts with protein ligation 15 , and solved structures of the substrate- and product-bound states. The structures show precise orientation of the megaenzyme preparing the nucleophilic attack of its key chemical step, and allow biochemical assays and quantum mechanical simulations to precisely interrogate the reaction. These data suggest that NRPSs C domains use a concerted reaction mechanism, where the active site histidine likely serves not a as general base, but as a crucial stabilizing hydrogen bond acceptor for the developing ammonium.
Organizational Affiliation:
Department of Biochemistry and Centre de recherche en biologie structurale, McGill University, Montréal, QC, Canada.