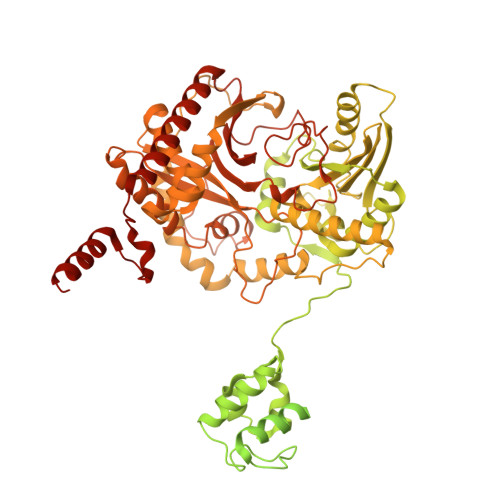
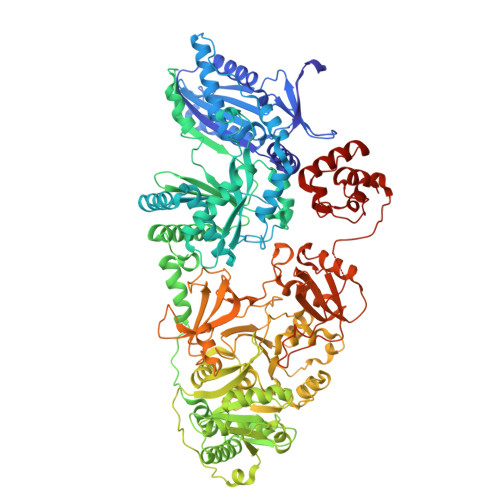
Crosslinking intermodular condensation in non-ribosomal peptide biosynthesis.
Heberlig, G.W., La Clair, J.J., Burkart, M.D.(2024) Nature
- PubMed: 39663458
- DOI: https://doi.org/10.1038/s41586-024-08306-y
- Primary Citation of Related Structures:
9BFD, 9BFE, 9BFF, 9BFG - PubMed Abstract:
Non-ribosomal peptide synthetases are assembly line biosynthetic pathways that are used to produce critical therapeutic drugs and are typically arranged as large multi-domain proteins called megasynthetases 1 . They synthesize polypeptides using peptidyl carrier proteins that shuttle each amino acid through modular loading, modification and elongation 2 steps, and remain challenging to structurally characterize, owing in part to the inherent dynamics of their multi-domain and multi-modular architectures 3 . Here we have developed site-selective crosslinking probes to conformationally constrain and resolve the interactions between carrier proteins and their partner enzymatic domains 4,5 . We apply tetrazine click chemistry to trap the condensation of two carrier protein substrates within the active site of the condensation domain that unites the first two modules of tyrocidine biosynthesis and report the high-resolution cryo-EM structure of this complex. Together with the X-ray crystal structure of the first carrier protein crosslinked to its epimerization domain, these structures highlight captured intermodular recognition events and define the processive movement of a carrier protein from one catalytic step to the next. Characterization of these structural relationships remains central to understanding the molecular details of these unique synthetases and critically informs future synthetic biology design of these pathways.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA, USA.