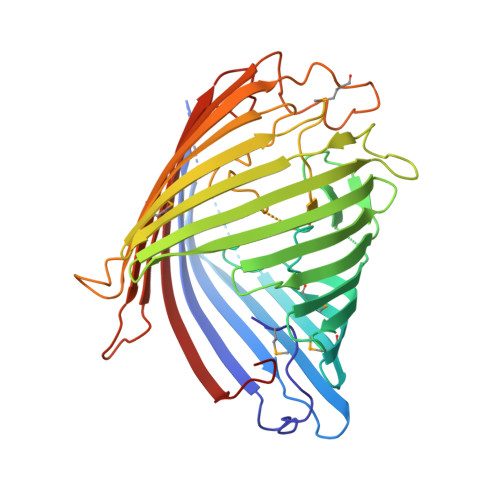
Structural insight into OprD substrate specificity.
Biswas, S., Mohammad, M.M., Patel, D.R., Movileanu, L., van den Berg, B.(2007) Nat Struct Mol Biol 14: 1108-1109
- PubMed: 17952093
- DOI: https://doi.org/10.1038/nsmb1304
- Primary Citation of Related Structures:
2ODJ - PubMed Abstract:
OprD proteins form a large family of substrate-specific outer-membrane channels in Gram-negative bacteria. We report here the X-ray crystal structure of OprD from Pseudomonas aeruginosa, which reveals a monomeric 18-stranded beta-barrel characterized by a very narrow pore constriction, with a positively charged basic ladder on one side and an electronegative pocket on the other side. The location of highly conserved residues in OprD suggests that the structure represents the general architecture of OprD channels.
Organizational Affiliation:
Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts 01605, USA.