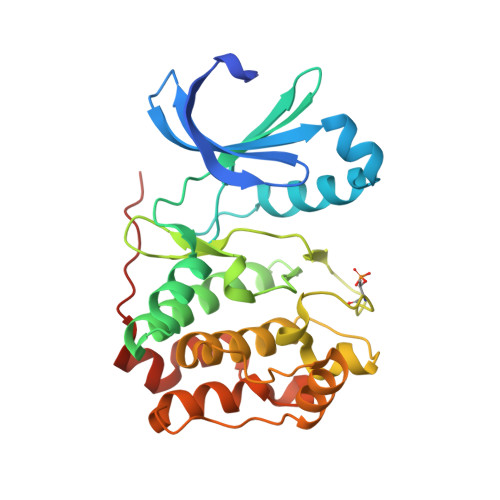
Reversine, a Novel Aurora Kinases Inhibitor, Inhibits Colony Formation of Human Acute Myeloid Leukemia Cells.
D'Alise, A.M., Amabile, G., Iovino, M., Di Giorgio, F.P., Bartiromo, M., Sessa, F., Villa, F., Musacchio, A., Cortese, R.(2008) Mol Cancer Ther 7: 1140
- PubMed: 18483302
- DOI: https://doi.org/10.1158/1535-7163.MCT-07-2051
- Primary Citation of Related Structures:
2VGO - PubMed Abstract:
The demonstration that the small synthetic molecule reversine [2-(4-morpholinoanilino)-N6-cyclohexyladenine] promotes the dedifferentiation of committed cells into multipotent progenitor-type cells has raised hopes on the exploitation of this small chemical tool for the generation of stem cells. Here, we show that reversine causes a failure in cytokinesis and induces polyploidization. These effects of reversine are due to the inhibition of Aurora A and B, two related kinases that are implicated in several aspects of mitosis and that are frequently amplified and overexpressed in human tumors. Reversine inhibits the phosphorylation of histone H3, a direct downstream target of Aurora kinases. Similarly to the Aurora kinase inhibitor VX-680, which has recently entered phase II clinical trials for cancer treatment, reversine inhibited colony formation of leukemic cells from patients with acute myeloid leukemia but was significantly less toxic than VX-680 on cells from healthy donors. The crystal structure of the reversine-Aurora B kinase complex shows that reversine is a novel class of ATP-competitive Aurora kinase inhibitors. Thus, although our studies raise serious doubts on the application of reversine in regenerative medicine, they support the paradigm that reversine might be a useful agent in cancer chemotherapy.
Organizational Affiliation:
CEINGE, Biotecnologie Avanzate, Via Comunale Margherita 482, 80131 Naples, Italy.