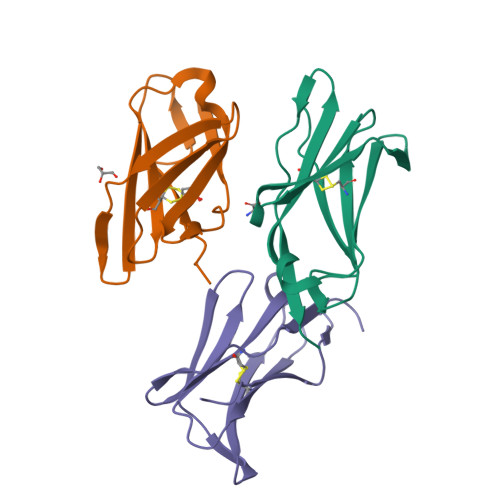
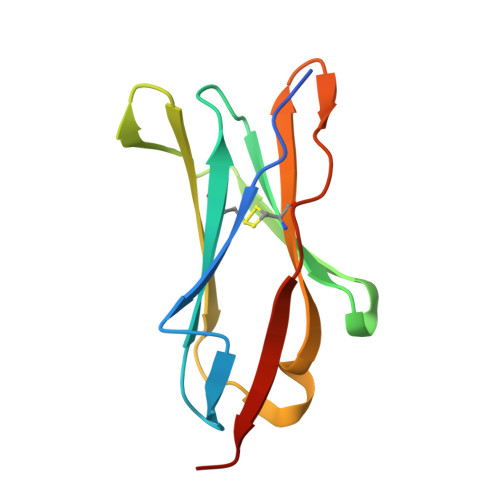
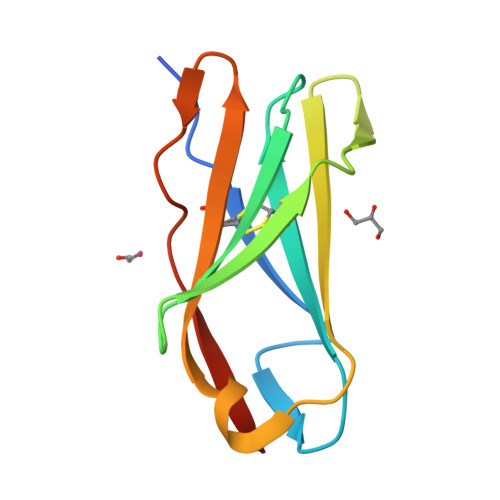
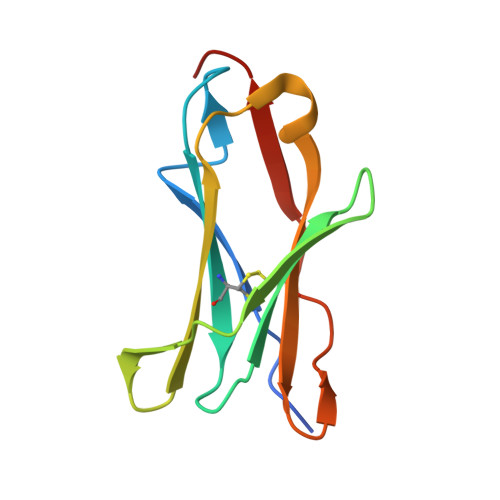
Crystal structure and collagen-binding site of immune inhibitory receptor LAIR-1: unexpected implications for collagen binding by platelet receptor GPVI
Brondijk, T.H.C., de Ruiter, T., Ballering, J., Wienk, H., Lebbink, R.J., van Ingen, H., Boelens, R., Farndale, R.W., Meyaard, L., Huizinga, E.G.(2010) Blood 115: 1364-1373
- PubMed: 20007810
- DOI: https://doi.org/10.1182/blood-2009-10-246322
- Primary Citation of Related Structures:
3KGR - PubMed Abstract:
Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1), one of the most widely spread immune receptors, attenuates immune cell activation when bound to specific sites in collagen. The collagen-binding domain of LAIR-1 is homologous to that of glycoprotein VI (GPVI), a collagen receptor crucial for platelet activation. Because LAIR-1 and GPVI also display overlapping collagen-binding specificities, a common structural basis for collagen recognition would appear likely. Therefore, it is crucial to gain insight into the molecular interaction of both receptors with their ligand to prevent unwanted cross-reactions during therapeutic intervention. We determined the crystal structure of LAIR-1 and mapped its collagen-binding site by nuclear magnetic resonance (NMR) titrations and mutagenesis. Our data identify R59, E61, and W109 as key residues for collagen interaction. These residues are strictly conserved in LAIR-1 and GPVI alike; however, they are located outside the previously proposed GPVI collagen-binding site. Our data provide evidence for an unanticipated mechanism of collagen recognition common to LAIR-1 and GPVI. This fundamental insight will contribute to the exploration of specific means of intervention in collagen-induced signaling in immunity and hemostasis.
Organizational Affiliation:
Crystal and Structural Chemistry, Bijvoet Center for Biomolecular Research, Utrecht University, Utrecht, The Netherlands.