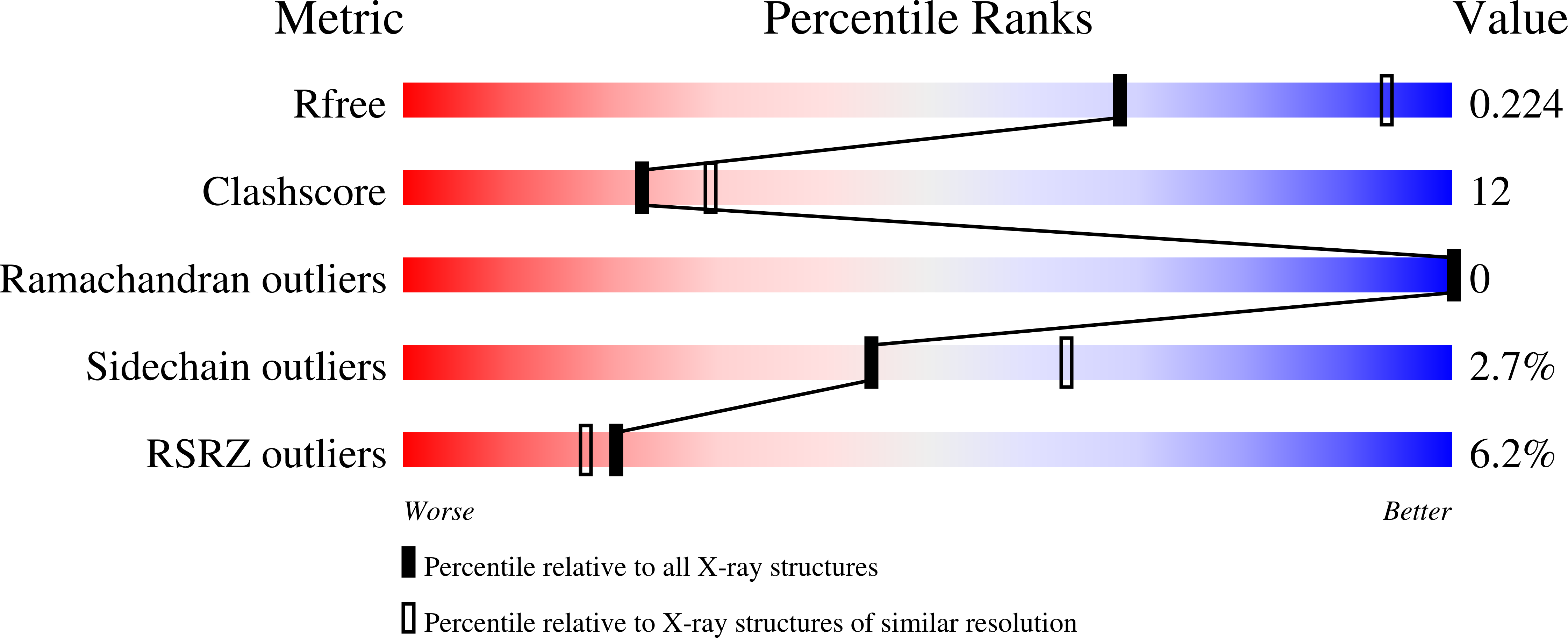
Insights into transport mechanism from LeuT engineered to transport tryptophan.
Piscitelli, C.L., Gouaux, E.(2012) EMBO J 31: 228-235
- PubMed: 21952050
- DOI: https://doi.org/10.1038/emboj.2011.353
- Primary Citation of Related Structures:
3QS4, 3QS5, 3QS6 - PubMed Abstract:
LeuT is a bacterial homologue of the neurotransmitter:sodium symporter (NSS) family and, being the only NSS member to have been structurally characterized by X-ray crystallography, is a model protein for studying transporter structure and mechanism. Transport activity in LeuT was hypothesized to require structural transitions between open-to-out and occluded conformations dependent upon protein:ligand binding complementarity. Here, using crystallographic and functional analysis, we show that binding site modification produces changes in both structure and activity that are consistent with complementarity-dependent structural transitions to the occluded state. The mutation I359Q converts the activity of tryptophan from inhibitor to transportable substrate. This mutation changes the local environment of the binding site, inducing the bound tryptophan to adopt a different conformer than in the wild-type complex. Instead of trapping the transporter open, tryptophan binding now allows the formation of an occluded state. Thus, transport activity is correlated to the ability of the ligand to promote the structural transition to the occluded state, a step in the transport cycle that is dependent on protein:ligand complementarity in the central binding site.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Oregon Health and Science University, Portland, OR 97239, USA.