Crystal Structures of PRK1 in Complex with the Clinical Compounds Lestaurtinib and Tofacitinib Reveal Ligand Induced Conformational Changes.
Chamberlain, P., Delker, S., Pagarigan, B., Mahmoudi, A., Jackson, P., Abbasian, M., Muir, J., Raheja, N., Cathers, B.(2014) PLoS One 9: e103638-e103638
- PubMed: 25111382
- DOI: https://doi.org/10.1371/journal.pone.0103638
- Primary Citation of Related Structures:
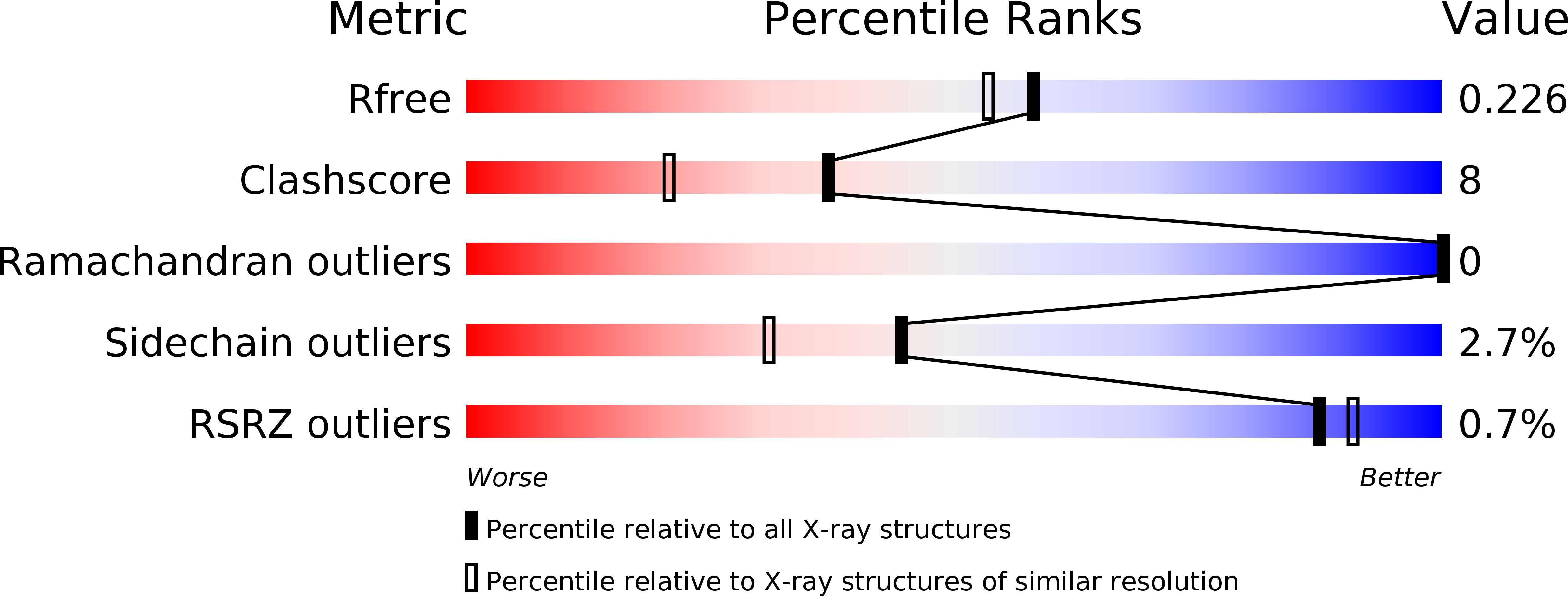
4OTD, 4OTG, 4OTH, 4OTI - PubMed Abstract:
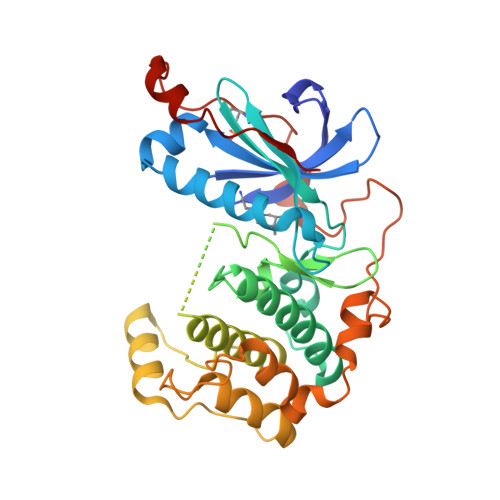
Protein kinase C related kinase 1 (PRK1) is a component of Rho-GTPase, androgen receptor, histone demethylase and histone deacetylase signaling pathways implicated in prostate and ovarian cancer. Herein we describe the crystal structure of PRK1 in apo form, and also in complex with a panel of literature inhibitors including the clinical candidates lestaurtinib and tofacitinib, as well as the staurosporine analog Ro-31-8220. PRK1 is a member of the AGC-kinase class, and as such exhibits the characteristic regulatory sequence at the C-terminus of the catalytic domain--the 'C-tail'. The C-tail fully encircles the catalytic domain placing a phenylalanine in the ATP-binding site. Our inhibitor structures include examples of molecules which both interact with, and displace the C-tail from the active site. This information may assist in the design of inhibitors targeting both PRK and other members of the AGC kinase family.
Organizational Affiliation:
Celgene Corporation, San Diego, California, United States of America; Department of Biochemistry and Structural Biology, Celgene Corporation, San Diego, California, United States of America.