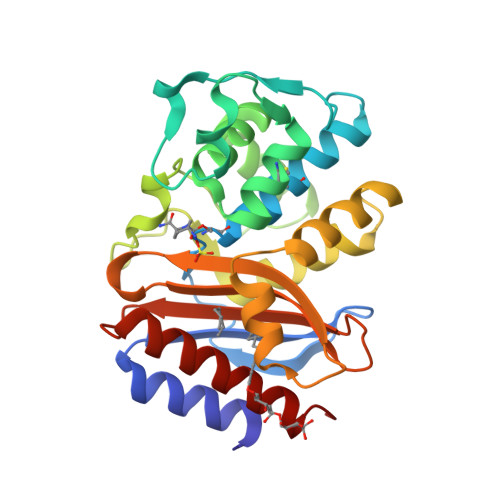
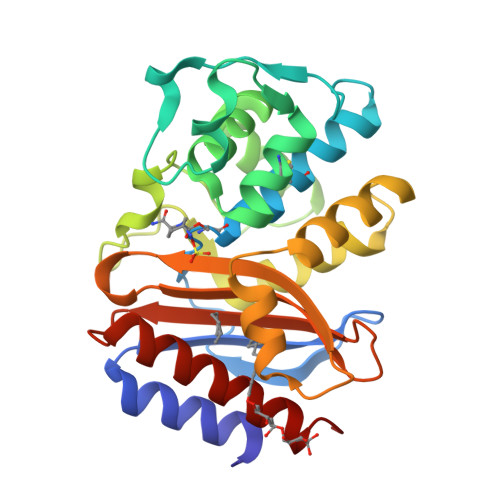
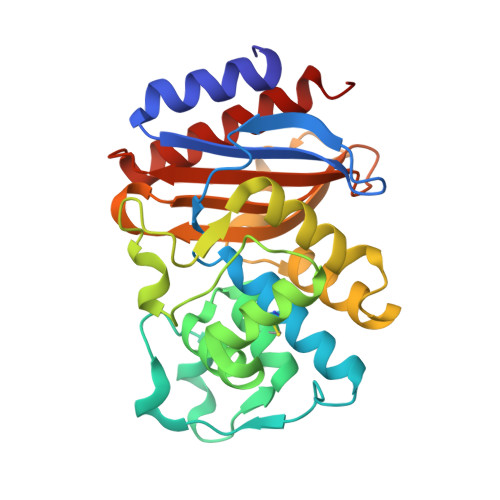
Inhibition of Klebsiella beta-Lactamases (SHV-1 and KPC-2) by Avibactam: A Structural Study.
Krishnan, N.P., Nguyen, N.Q., Papp-Wallace, K.M., Bonomo, R.A., van den Akker, F.(2015) PLoS One 10: e0136813-e0136813
- PubMed: 26340563
- DOI: https://doi.org/10.1371/journal.pone.0136813
- Primary Citation of Related Structures:
4ZAM, 4ZBE - PubMed Abstract:
β-Lactamase inhibition is an important clinical strategy in overcoming β-lactamase-mediated resistance to β-lactam antibiotics in Gram negative bacteria. A new β-lactamase inhibitor, avibactam, is entering the clinical arena and promising to be a major step forward in our antibiotic armamentarium. Avibactam has remarkable broad-spectrum activity in being able to inhibit classes A, C, and some class D β-lactamases. We present here structural investigations into class A β-lactamase inhibition by avibactam as we report the crystal structures of SHV-1, the chromosomal penicillinase of Klebsiella pneumoniae, and KPC-2, an acquired carbapenemase found in the same pathogen, complexed with avibactam. The 1.80 Å KPC-2 and 1.42 Å resolution SHV-1 β-lactamase avibactam complex structures reveal avibactam covalently bonded to the catalytic S70 residue. Analysis of the interactions and chair-shaped conformation of avibactam bound to the active sites of KPC-2 and SHV-1 provides structural insights into recently laboratory-generated amino acid substitutions that result in resistance to avibactam in KPC-2 and SHV-1. Furthermore, we observed several important differences in the interactions with amino acid residues, in particular that avibactam forms hydrogen bonds to S130 in KPC-2 but not in SHV-1, that can possibly explain some of the different kinetic constants of inhibition. Our observations provide a possible reason for the ability of KPC-2 β-lactamase to slowly desulfate avibactam with a potential role for the stereochemistry around the N1 atom of avibactam and/or the presence of an active site water molecule that could aid in avibactam desulfation, an unexpected consequence of novel inhibition chemistry.
Organizational Affiliation:
Department of Biochemistry, Case Western Reserve University, 10900 Euclid Ave., Cleveland, OH, 44106, United States of America.