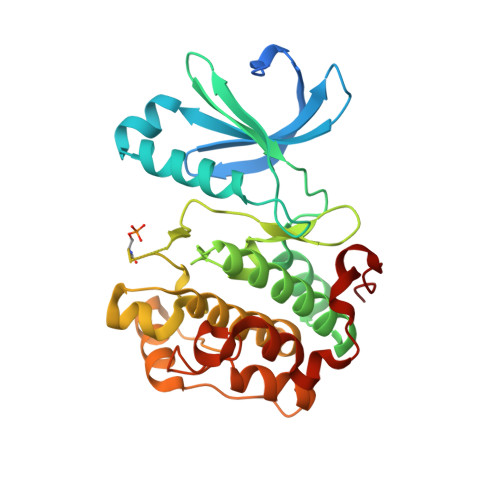
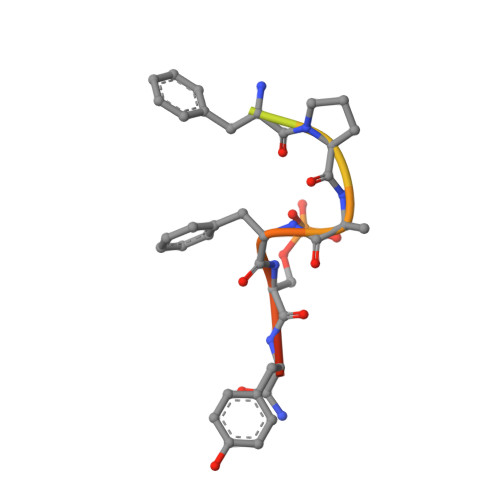
Bidirectional Allosteric Communication between the ATP-Binding Site and the Regulatory PIF Pocket in PDK1 Protein Kinase.
Schulze, J.O., Saladino, G., Busschots, K., Neimanis, S., Su, E., Odadzic, D., Zeuzem, S., Hindie, V., Herbrand, A.K., Lisa, M.N., Alzari, P.M., Gervasio, F.L., Biondi, R.M.(2016) Cell Chem Biol 23: 1193-1205
- PubMed: 27693059
- DOI: https://doi.org/10.1016/j.chembiol.2016.06.017
- Primary Citation of Related Structures:
5LVL, 5LVM, 5LVN, 5LVO, 5LVP - PubMed Abstract:
Allostery is a phenomenon observed in many proteins where binding of a macromolecular partner or a small-molecule ligand at one location leads to specific perturbations at a site not in direct contact with the region where the binding occurs. The list of proteins under allosteric regulation includes AGC protein kinases. AGC kinases have a conserved allosteric site, the phosphoinositide-dependent protein kinase 1 (PDK1)-interacting fragment (PIF) pocket, which regulates protein ATP-binding, activity, and interaction with substrates. In this study, we identify small molecules that bind to the ATP-binding site and affect the PIF pocket of AGC kinase family members, PDK1 and Aurora kinase. We describe the mechanistic details and show that although PDK1 and Aurora kinase inhibitors bind to the conserved ATP-binding site, they differentially modulate physiological interactions at the PIF-pocket site. Our work outlines a strategy for developing bidirectional small-molecule allosteric modulators of protein kinases and other signaling proteins.
Organizational Affiliation:
Research Group PhosphoSites, Department of Internal Medicine I, Universitätsklinikum Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany.