Crystal structure and mechanistic analysis of a novel human kynurenine aminotransferase-2 reversible inhibitor
Nematollahi, A., Sun, G., Jayawickrama, G., Hanrahan, J.R., Church, W.B.(2017) Med Chem Res
Experimental Data Snapshot
Starting Model: experimental
View more details
(2017) Med Chem Res
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial | 425 | Homo sapiens | Mutation(s): 0 Gene Names: AADAT, KAT2 EC: 2.6.1.39 (PDB Primary Data), 2.6.1.7 (PDB Primary Data), 2.6.1.4 (UniProt), 2.6.1.63 (UniProt), 2.6.1.73 (UniProt) | 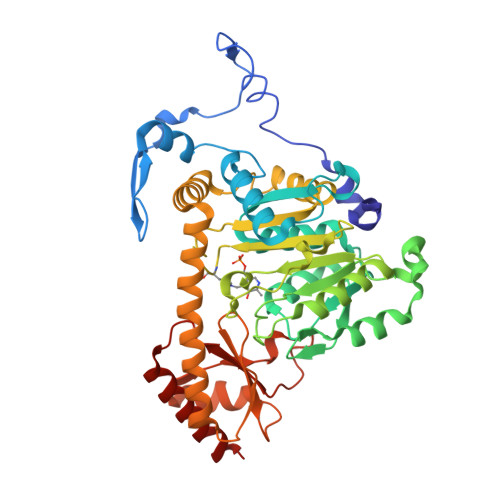 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8N5Z0 (Homo sapiens) Explore Q8N5Z0 Go to UniProtKB: Q8N5Z0 | |||||
PHAROS: Q8N5Z0 GTEx: ENSG00000109576 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8N5Z0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 7AR Query on 7AR | C [auth B] | (2R)-2-(5,6-dichloro-1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-phenylpropanoic acid C17 H11 Cl2 N O4 MYKVEESSMOYIFU-CQSZACIVSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| LLP Query on LLP | A, B | L-PEPTIDE LINKING | C14 H22 N3 O7 P |  | LYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 88.883 | α = 90 |
| b = 99.275 | β = 90 |
| c = 107.923 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Blu-Ice | data collection |
| PHASER | phasing |