Enhancement of therapeutic potential of a naturally occurring human antibody targeting a phosphorylated Ser422containing epitope on pathological tau.
van Ameijde, J., Crespo, R., Janson, R., Juraszek, J., Siregar, B., Verveen, H., Sprengers, I., Nahar, T., Hoozemans, J.J., Steinbacher, S., Willems, R., Delbroek, L., Borgers, M., Dockx, K., Van Kolen, K., Mercken, M., Pascual, G., Koudstaal, W., Apetri, A.(2018) Acta Neuropathol Commun 6: 59-59
- PubMed: 30001207
- DOI: https://doi.org/10.1186/s40478-018-0562-9
- Primary Citation of Related Structures:
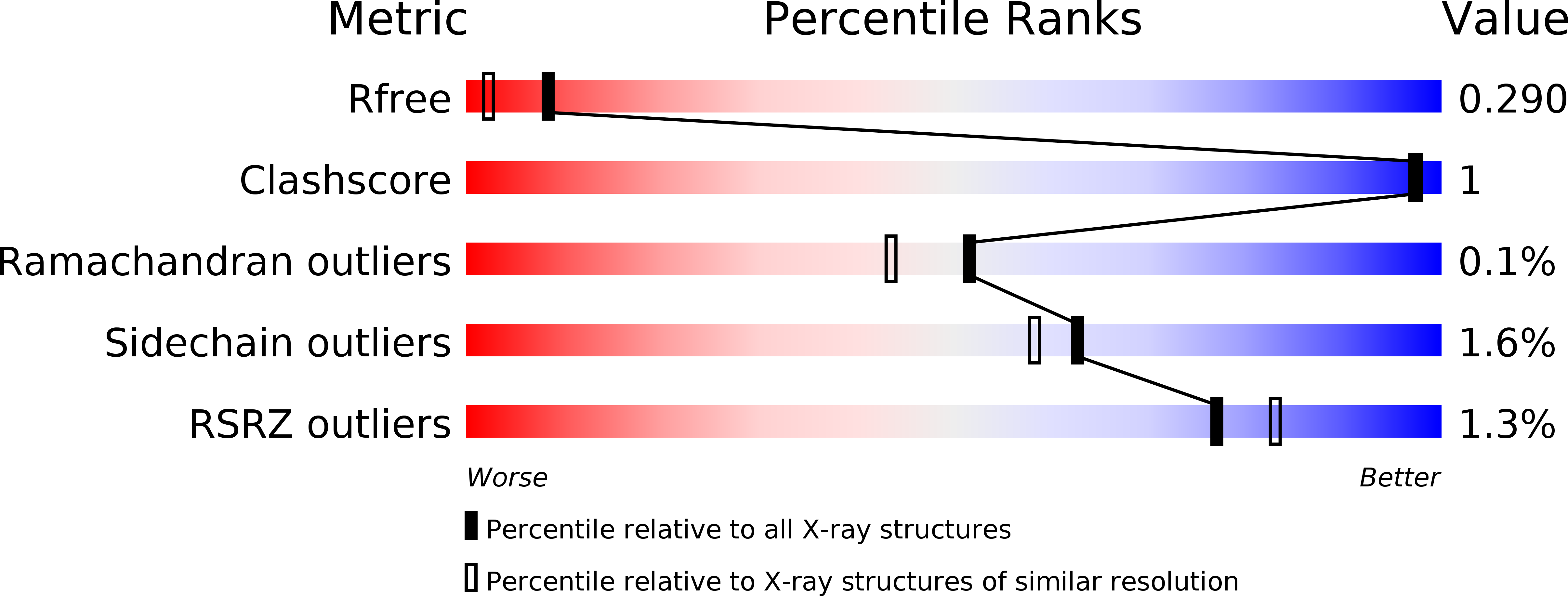
6H06, 6H0E - PubMed Abstract:
Aggregation of tau protein and spreading of tau aggregates are pivotal pathological processes in a range of neurological disorders. Accumulating evidence suggests that immunotherapy targeting tau may be a viable therapeutic strategy. We have previously described the isolation of antibody CBTAU-22.1 from the memory B-cell repertoire of healthy human donors. CBTAU-22.1 was shown to specifically bind a disease-associated phosphorylated epitope in the C-terminus of tau (Ser 422 ) and to be able to inhibit the spreading of pathological tau aggregates from P301S spinal cord lysates in vitro, albeit with limited potency. Using a combination of rational design and random mutagenesis we have derived a variant antibody with improved affinity while maintaining the specificity of the parental antibody. This affinity improved antibody showed greatly enhanced potency in a cell-based immunodepletion assay using paired helical filaments (PHFs) derived from human Alzheimer's disease (AD) brain tissue. Moreover, the affinity improved antibody limits the in vitro aggregation propensity of full length tau species specifically phosphorylated at position 422 produced by employing a native chemical ligation approach. Together, these results indicate that in addition to being able to inhibit the spreading of pathological tau aggregates, the matured antibody can potentially also interfere with the nucleation of tau which is believed to be the first step of the pathogenic process. Finally, the functionality in a P301L transgenic mice co-injection model highlights the therapeutic potential of human antibody dmCBTAU-22.1.
Organizational Affiliation:
Janssen Prevention Center, Janssen Pharmaceutical Companies of Johnson and Johnson, Archimedesweg 6, 2333, Leiden, CN, the Netherlands.