Molecular features of the UNC-45 chaperone critical for binding and folding muscle myosin.
Hellerschmied, D., Lehner, A., Franicevic, N., Arnese, R., Johnson, C., Vogel, A., Meinhart, A., Kurzbauer, R., Deszcz, L., Gazda, L., Geeves, M., Clausen, T.(2019) Nat Commun 10: 4781-4781
- PubMed: 31636255
- DOI: https://doi.org/10.1038/s41467-019-12667-8
- Primary Citation of Related Structures:
6QDJ, 6QDK, 6QDL, 6QDM - PubMed Abstract:
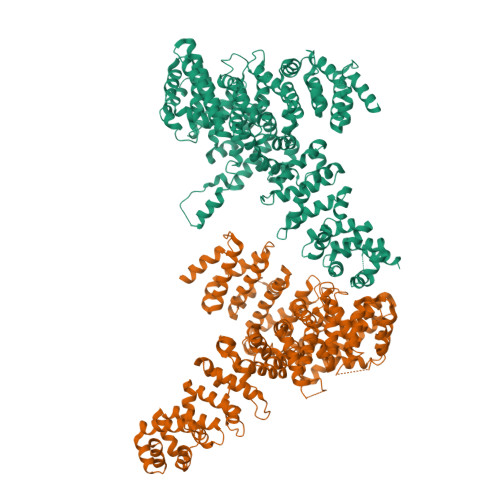
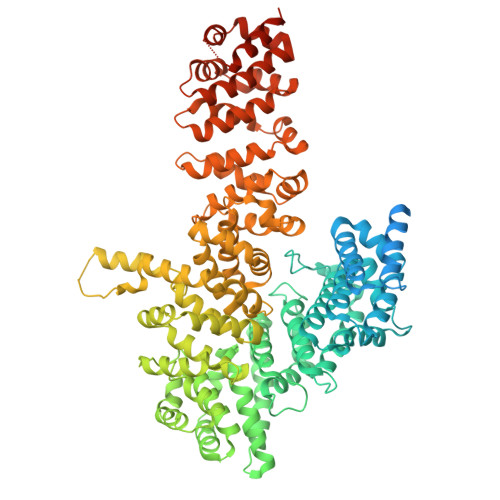
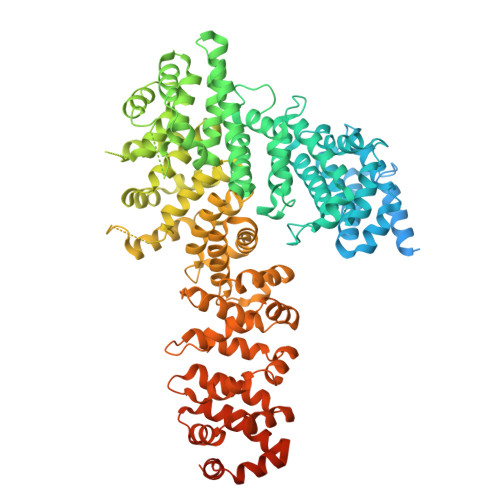
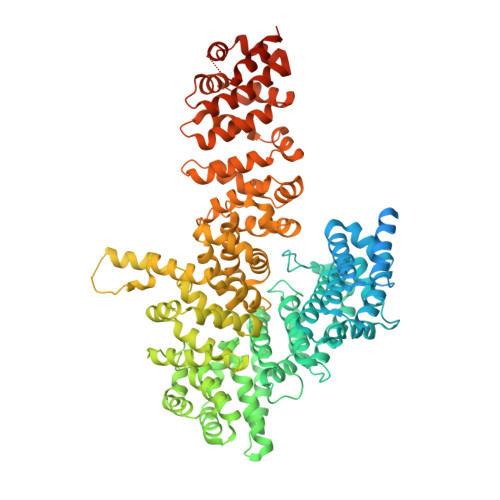
Myosin is a motor protein that is essential for a variety of processes ranging from intracellular transport to muscle contraction. Folding and assembly of myosin relies on a specific chaperone, UNC-45. To address its substrate-targeting mechanism, we reconstitute the interplay between Caenorhabditis elegans UNC-45 and muscle myosin MHC-B in insect cells. In addition to providing a cellular chaperone assay, the established system enabled us to produce large amounts of functional muscle myosin, as evidenced by a biochemical and structural characterization, and to directly monitor substrate binding to UNC-45. Data from in vitro and cellular chaperone assays, together with crystal structures of binding-deficient UNC-45 mutants, highlight the importance of utilizing a flexible myosin-binding domain. This so-called UCS domain can adopt discrete conformations to efficiently bind and fold substrate. Moreover, our data uncover the molecular basis of temperature-sensitive UNC-45 mutations underlying one of the most prominent motility defects in C. elegans.
Organizational Affiliation:
Research Institute of Molecular Pathology, Vienna BioCenter, Vienna, Austria. doris.hellerschmied@uni-due.de.