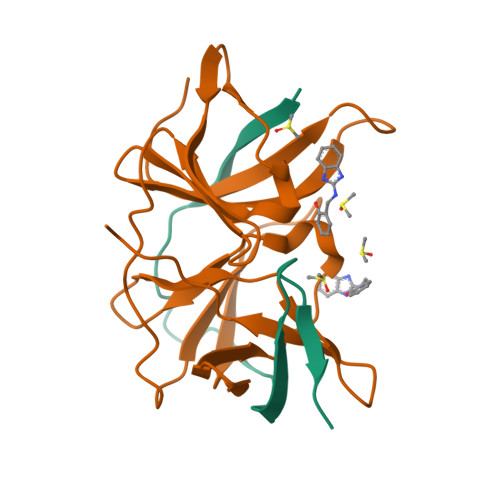
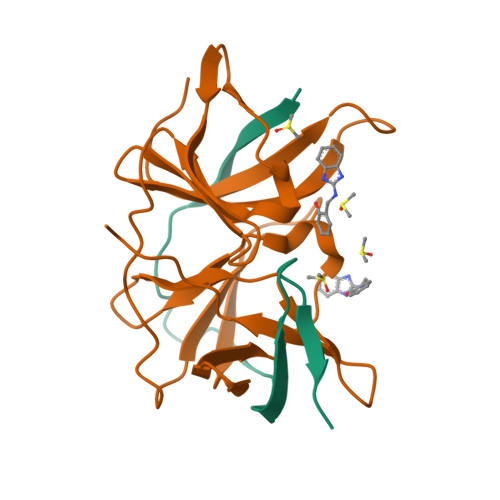
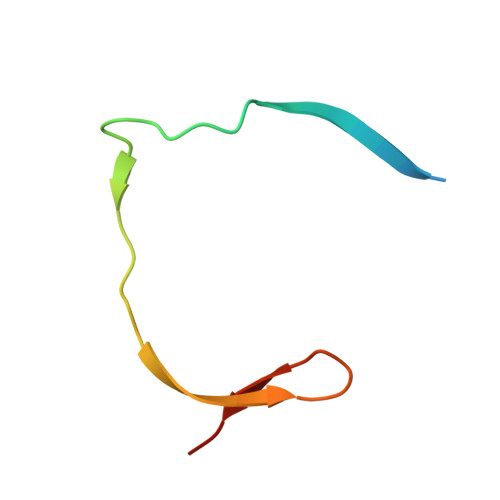
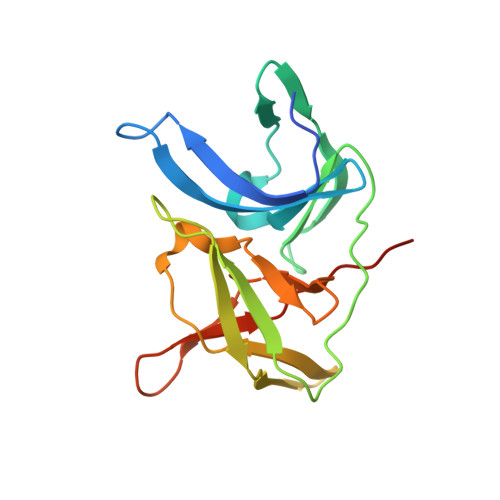
PanDDA analysis group deposition of ZIKV NS2B-NS3 protease
Ni, X., Godoy, A.S., Marples, P.G., Fairhead, M., Balcomb, B.H., Tomlinson, C.W.E., Koekemoer, L., Aschenbrenner, J.C., Lithgo, R.M., Thompson, W., Wild, C., Williams, E.P., Winokan, M., Chandran, A.V., Fearon, D., Walsh, M.A., von Delft, F.To be published.