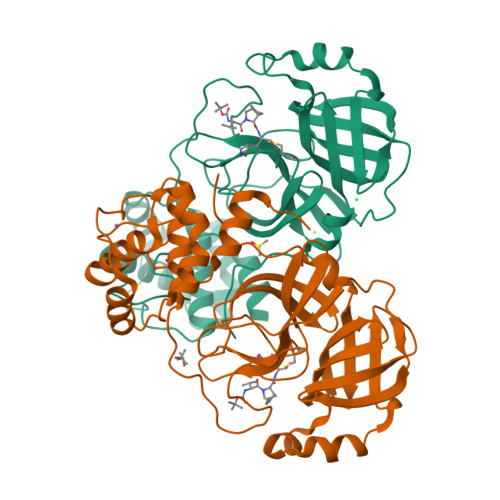
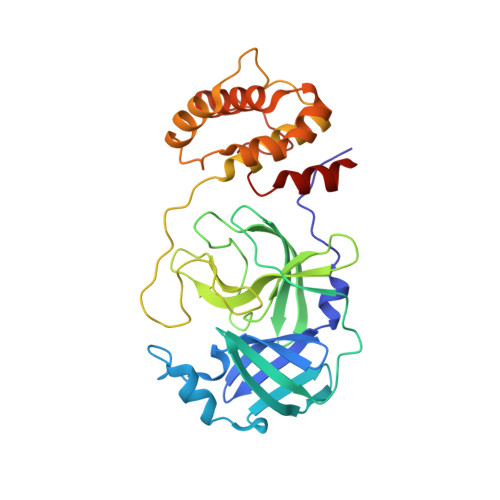
Broad-spectrum coronavirus 3C-like protease peptidomimetic inhibitors effectively block SARS-CoV-2 replication in cells: Design, synthesis, biological evaluation, and X-ray structure determination.
Stefanelli, I., Corona, A., Cerchia, C., Cassese, E., Improta, S., Costanzi, E., Pelliccia, S., Morasso, S., Esposito, F., Paulis, A., Scognamiglio, S., Di Leva, F.S., Storici, P., Brindisi, M., Tramontano, E., Cannalire, R., Summa, V.(2023) Eur J Med Chem 253: 115311-115311
- PubMed: 37043904
- DOI: https://doi.org/10.1016/j.ejmech.2023.115311
- Primary Citation of Related Structures:
8OKK, 8OKL, 8OKM, 8OKN - PubMed Abstract:
Despite the approval of vaccines, monoclonal antibodies and restrictions during the pandemic, the demand for new efficacious and safe antivirals is compelling to boost the therapeutic arsenal against the COVID-19. The viral 3-chymotrypsin-like protease (3CL pro ) is an essential enzyme for replication with high homology in the active site across CoVs and variants showing an almost unique specificity for Leu-Gln as P2-P1 residues, allowing the development of broad-spectrum inhibitors. The design, synthesis, biological activity, and cocrystal structural information of newly conceived peptidomimetic covalent reversible inhibitors are herein described. The inhibitors display an aldehyde warhead, a Gln mimetic at P1 and modified P2-P3 residues. Particularly, functionalized proline residues were inserted at P2 to stabilize the β-turn like bioactive conformation, modulating the affinity. The most potent compounds displayed low/sub-nM potency against the 3CL pro of SARS-CoV-2 and MERS-CoV and inhibited viral replication of three human CoVs, i.e. SARS-CoV-2, MERS-CoV, and HCoV 229 in different cell lines. Particularly, derivative 12 exhibited nM-low μM antiviral activity depending on the virus, and the highest selectivity index. Some compounds were co-crystallized with SARS-CoV-2 3CL pro validating our design. Altogether, these results foster future work toward broad-spectrum 3CL pro inhibitors to challenge CoVs related pandemics.
Organizational Affiliation:
Department of Pharmacy, University of Naples Federico II, via D. Montesano 49, 80131, Naples, Italy.