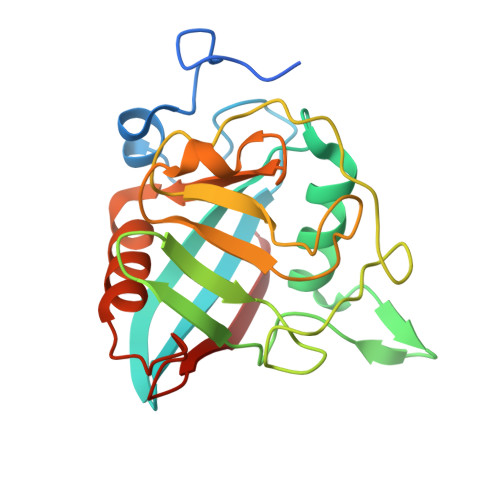
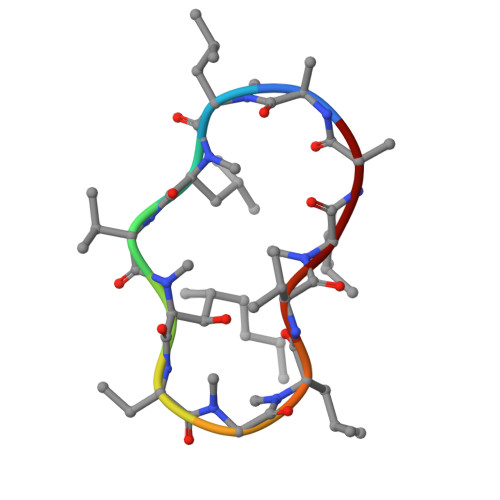
Evaluating the potential of non-immunosuppressive cyclosporin analogs for targeting Toxoplasma gondii cyclophilin: Insights from structural studies.
Favretto, F., Jimenez-Faraco, E., Catucci, G., Di Matteo, A., Travaglini-Allocatelli, C., Sadeghi, S.J., Dominici, P., Hermoso, J.A., Astegno, A.(2024) Protein Sci 33: e5157-e5157
- PubMed: 39312281
- DOI: https://doi.org/10.1002/pro.5157
- Primary Citation of Related Structures:
8R7S, 8R7T, 8R7U - PubMed Abstract:
Toxoplasmosis persists as a prevalent disease, facing challenges from parasite resistance and treatment side effects. Consequently, identifying new drugs by exploring novel protein targets is essential for effective intervention. Cyclosporin A (CsA) possesses antiparasitic activity against Toxoplasma gondii, with cyclophilins identified as possible targets. However, CsA immunosuppressive nature hinders its use as an antitoxoplasmosis agent. Here, we evaluate the potential of three CsA derivatives devoid of immunosuppressive activity, namely, NIM811, Alisporivir, and dihydrocyclosporin A to target a previously characterized cyclophilin from Toxoplasma gondii (TgCyp23). We determined the X-ray crystal structures of TgCyp23 in complex with the three analogs and elucidated their binding and inhibitory properties. The high resolution of the structures revealed the precise positioning of ligands within the TgCyp23 binding site and the details of protein-ligand interactions. A comparison with the established ternary structure involving calcineurin indicates that substitutions at position 4 in CsA derivatives prevent calcineurin binding. This finding provides a molecular explanation for why CsA analogs can target Toxoplasma cyclophilins without compromising the human immune response.
Organizational Affiliation:
Department of Biotechnology, University of Verona, Verona, Italy.