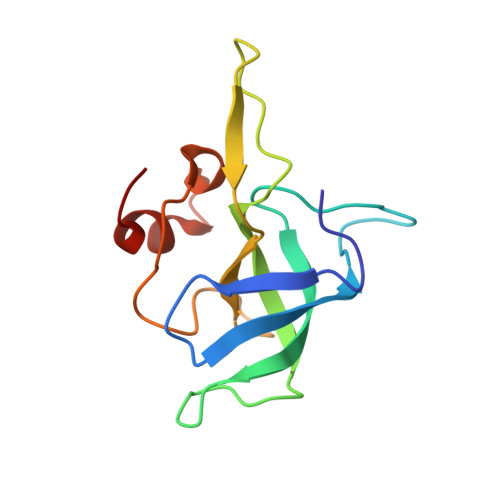
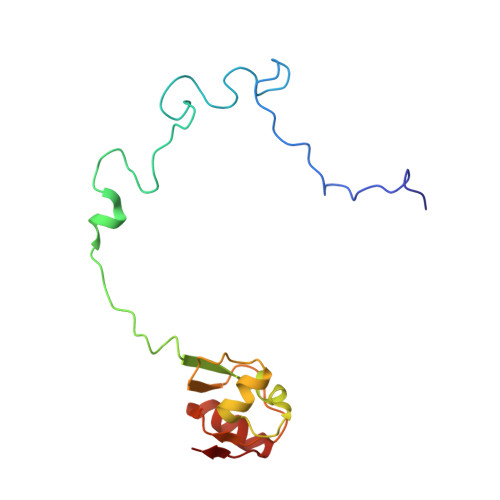
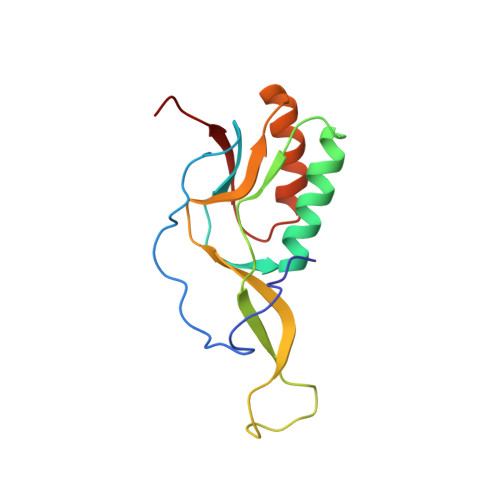
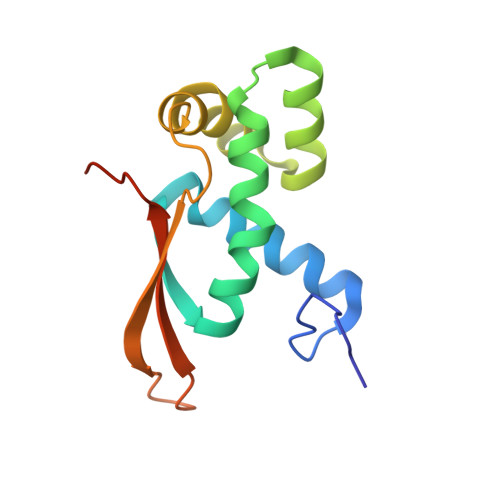
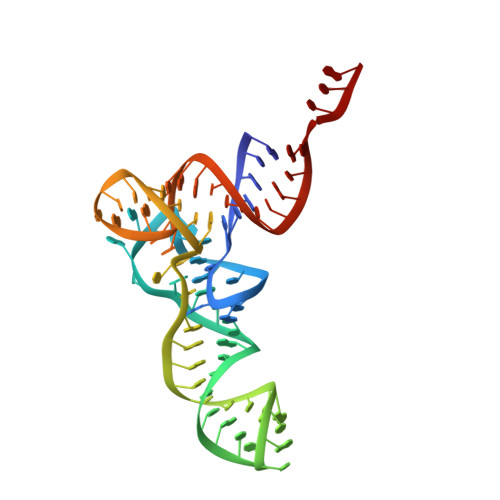The Myxobacterial Antibiotic Myxovalargin: Biosynthesis, Structural Revision, Total Synthesis, and Molecular Characterization of Ribosomal Inhibition.
Koller, T.O., Scheid, U., Kosel, T., Herrmann, J., Krug, D., Boshoff, H.I.M., Beckert, B., Evans, J.C., Schlemmer, J., Sloan, B., Weiner, D.M., Via, L.E., Moosa, A., Ioerger, T.R., Graf, M., Zinshteyn, B., Abdelshahid, M., Nguyen, F., Arenz, S., Gille, F., Siebke, M., Seedorf, T., Plettenburg, O., Green, R., Warnke, A.L., Ullrich, J., Warrass, R., Barry 3rd, C.E., Warner, D.F., Mizrahi, V., Kirschning, A., Wilson, D.N., Muller, R.(2023) J Am Chem Soc 145: 851-863
- PubMed: 36603206
- DOI: https://doi.org/10.1021/jacs.2c08816
- Primary Citation of Related Structures:
7QQ3, 8B7Y - PubMed Abstract:
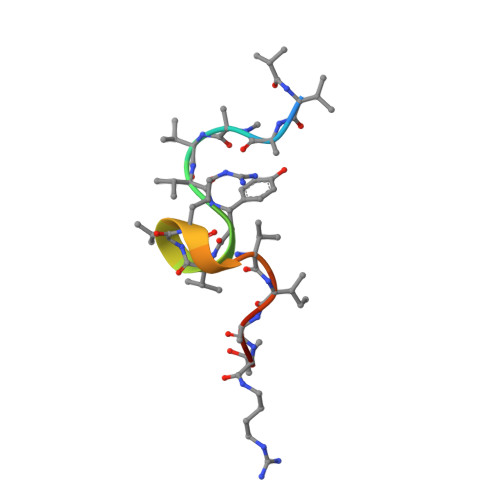
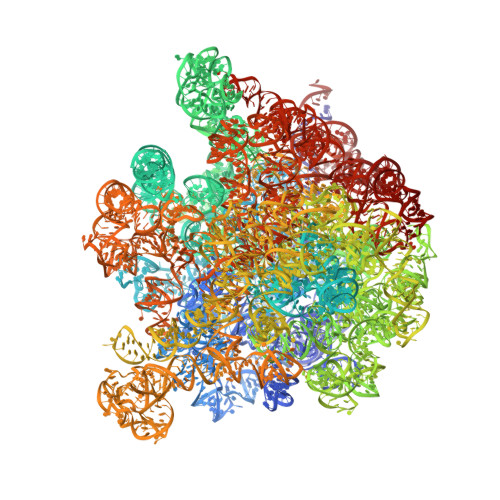
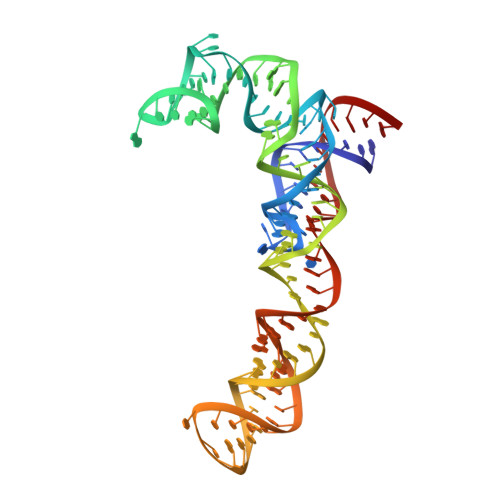
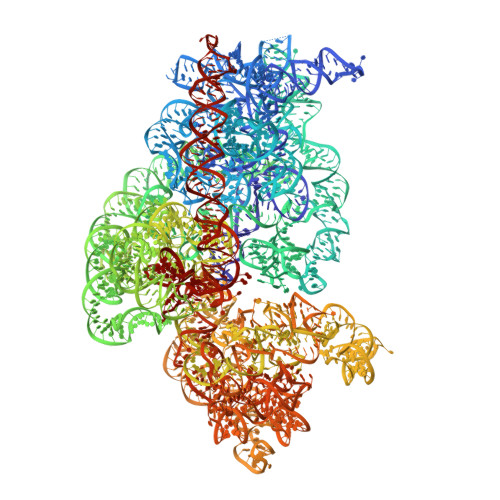
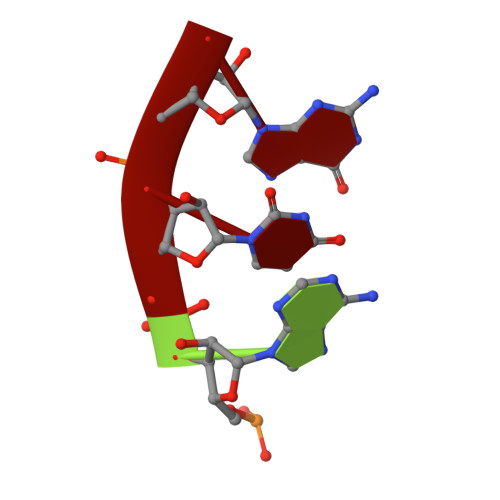
Resistance of bacterial pathogens against antibiotics is declared by WHO as a major global health threat. As novel antibacterial agents are urgently needed, we re-assessed the broad-spectrum myxobacterial antibiotic myxovalargin and found it to be extremely potent against Mycobacterium tuberculosis . To ensure compound supply for further development, we studied myxovalargin biosynthesis in detail enabling production via fermentation of a native producer. Feeding experiments as well as functional genomics analysis suggested a structural revision, which was eventually corroborated by the development of a concise total synthesis. The ribosome was identified as the molecular target based on resistant mutant sequencing, and a cryo-EM structure revealed that myxovalargin binds within and completely occludes the exit tunnel, consistent with a mode of action to arrest translation during a late stage of translation initiation. These studies open avenues for structure-based scaffold improvement toward development as an antibacterial agent.
Organizational Affiliation:
Institute for Biochemistry and Molecular Biology, University of Hamburg, Martin-Luther-King-Platz 6, 20146 Hamburg, Germany.